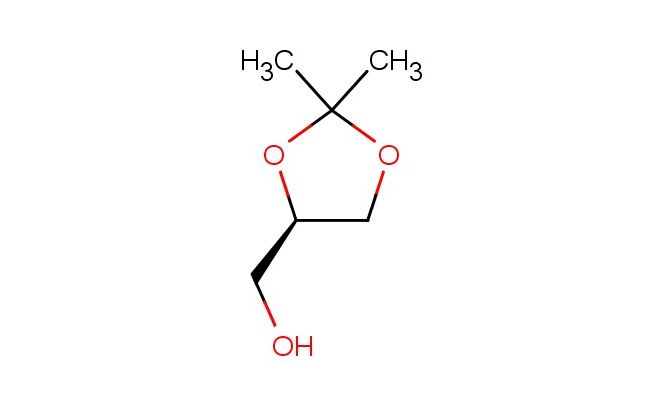
(R)-(2,2-dimethyl-1,3-dioxolan-4-yl)methanol
$350.00
CAS No.: 14347-78-5
Catalog No.: 196129
Purity: 95%
MF: C6H12O3
MW: 132.159
Storage: 2-8 degree Celsius
SMILES: CC1(OC[C@H](O1)CO)C
Catalog No.: 196129
Purity: 95%
MF: C6H12O3
MW: 132.159
Storage: 2-8 degree Celsius
SMILES: CC1(OC[C@H](O1)CO)C
(R)-(2,2-dimethyl-1,3-dioxolan-4-yl)methanol; CAS No.: 14347-78-5; (R)-(2,2-dimethyl-1,3-dioxolan-4-yl)methanol. PROPERTIES: This colorless liquid has a molecular formula of C6H10O3 and a molecular weight of approximately 130.14 g/mol. It exhibits good solubility in water and common organic solvents. The compound is sensitive to acidic conditions and should be stored in a tightly sealed container at room temperature. Thermogravimetric analysis shows decomposition starting at 150 C. Safety guidelines recommend using chemical-resistant gloves, splash goggles, and working in a well-ventilated area. In case of accidental ingestion, rinse mouth and seek immediate medical advice. Avoid release to the environment as it may be harmful to aquatic organisms. APPLICATIONS: (R)-(2,2-dimethyl-1,3-dioxolan-4-yl)methanol serves as a chiral auxiliary in asymmetric synthesis, particularly in the pharmaceutical industry. Its dioxolane core provides conformational constraints useful in controlling stereochemistry during reactions. The hydroxyl group enables further functionalization through ether formation or oxidation. Research groups employ it in the synthesis of enantiomerically pure beta-amino alcohols for drug discovery. Academic institutions utilize it in teaching asymmetric synthesis and chiral resolution techniques. Industrial applications include its use as a chiral building block in agrochemical development for selective herbicide and fungicide candidates. Recent publications in Tetrahedron: Asymmetry highlight its role in enantioselective organocatalytic reactions. Additionally, it finds utility in materials science as a chiral dopant in liquid crystal formulations. The compound's ability to form stable crystalline derivatives makes it suitable for chiral purity determination studies. Its biodegradability profile makes it preferable to persistent chiral auxiliaries in green chemistry initiatives.
Reviews
Write Your Own Review