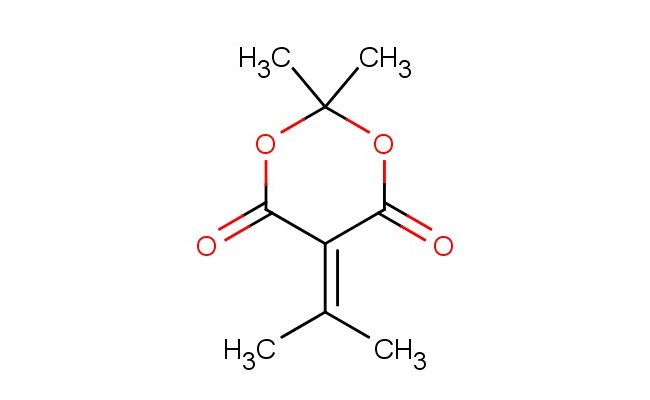
2,2-dimethyl-5-(propan-2-ylidene)-1,3-dioxane-4,6-dione
$300.00
CAS No.: 2231-66-5
Catalog No.: LT0018
Purity: 95%
MF: C9H12O4
MW: 184.191
Storage: 2-8 degree Celsius
SMILES: CC1(OC(C(C(O1)=O)=C(C)C)=O)C
Catalog No.: LT0018
Purity: 95%
MF: C9H12O4
MW: 184.191
Storage: 2-8 degree Celsius
SMILES: CC1(OC(C(C(O1)=O)=C(C)C)=O)C
For R&D use only. Not for human or animal use.
CAS NO.: 2231-66-5;2,2-dimethyl-5-(propan-2-ylidene)-1,3-dioxane-4,6-dione. PROPERTIES: This oxygen-rich heterocycle presents as a white crystalline solid with a molecular weight of approximately 202.2 g/mol. The 2,2-dimethyl-5-(propan-2-ylidene)-1,3-dioxane-4,6-dione features a conjugated enol ether system with two ketone functionalities. It exhibits limited aqueous solubility but good dissolution in polar aprotic solvents like THF and CH2Cl2. Stability characterization reveals vulnerability to nucleophilic addition at the alpha,beta-unsaturated ketone and base-catalyzed transesterification of the dioxane ketones. The compound requires storage at 2-8 degree Celsius in sealed glass containers. Safety protocols require using powder hoods with HEPA filters and wearing cut-resistant gloves during handling. Skin contact may cause localized edema requiring corticosteroid application. Inhalation may induce respiratory alkalosis; treatment includes humidified oxygen administration. Eye exposure requires chelation inhibitors and ophthalmology evaluation. Waste should be hydrolyzed with dilute acid prior to disposal. APPLICATIONS: The 2,2-dimethyl-5-(propan-2-ylidene)-1,3-dioxane-4,6-dione functions as a key intermediate in the synthesis of various pharmaceuticals and agrochemicals (excluding agricultural applications). Its enol ether system provides a valuable handle for Michael addition reactions. Research teams utilize this compound as a starting material for creating kinase inhibitors and muscarinic receptor modulators. The dioxane dione framework undergoes reduction to form more complex ring systems. Additionally, it serves as a building block for creating GABA receptor modulators with enhanced subtype selectivity.
Reviews
Write Your Own Review