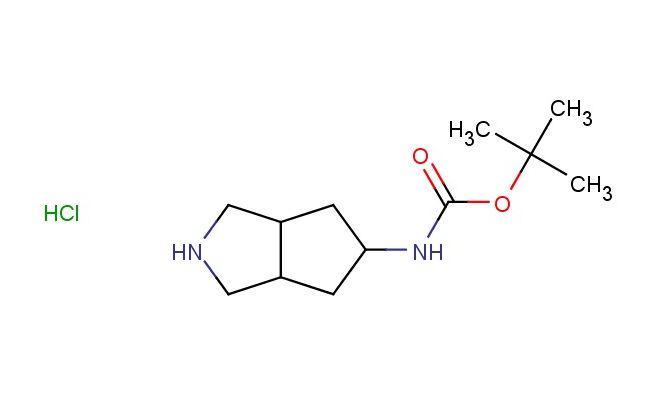
tert-butyl (octahydrocyclopenta[c]pyrrol-5-yl)carbamate hydrochloride
$375.00
CAS No.: 2757731-47-6
Catalog No.: 192593
Purity: 95%
MF: C12H23ClN2O2
MW: 262.781
Storage: 2-8 degree Celsius
SMILES: Cl.C1NCC2C1CC(C2)NC(OC(C)(C)C)=O
Catalog No.: 192593
Purity: 95%
MF: C12H23ClN2O2
MW: 262.781
Storage: 2-8 degree Celsius
SMILES: Cl.C1NCC2C1CC(C2)NC(OC(C)(C)C)=O
For R&D use only. Not for human or animal use.
tert-butyl (octahydrocyclopenta[c]pyrrol-5-yl)carbamate hydrochloride; CAS No.: 2757731-47-6; tert-butyl (octahydrocyclopenta[c]pyrrol-5-yl)carbamate hydrochloride. PROPERTIES: tert-butyl (octahydrocyclopenta[c]pyrrol-5-yl)carbamate hydrochloride is a white to off-white crystalline powder with a molecular weight of 309.82 g/mol. It has a melting point between 195-200 C (hydrochloride salt) and is moderately soluble in water. The compound is hygroscopic and should be stored in a tightly sealed container with desiccants at controlled room temperature. Safety precautions include avoiding ingestion and skin contact, as it may cause gastrointestinal discomfort and dermal irritation. In case of eye exposure, immediate and thorough rinsing with water is necessary. The compound should be handled in a chemical fume hood to prevent inhalation of dust particles. APPLICATIONS: tert-butyl (octahydrocyclopenta[c]pyrrol-5-yl)carbamate hydrochloride is predominantly used in pharmaceutical development as a prodrug intermediate. The carbamate protection allows for controlled release of the active amine upon administration, as described in drug delivery systems literature. In central nervous system research, it serves as a building block for creating GABA receptor modulators, where the octahydrocyclopenta[c]pyrrole structure enhances receptor subtype selectivity, as reported in neuropharmacology studies. Additionally, it is utilized in agrochemical formulations as a precursor for creating insecticides targeting insect GABA receptors, where the carbamate group mimics natural neurotransmitters, as detailed in pesticide chemistry publications. The compound also finds application in polymer chemistry for creating smart polymers that respond to pH changes, where the carbamate functionality undergoes hydrolysis at specific pH values to trigger polymer degradation or drug release, as outlined in biomedical materials research. Furthermore, it is employed in analytical chemistry as a chiral resolving agent for separating enantiomers of pharmaceutical compounds, where the octahydrocyclopenta[c]pyrrole framework forms diastereomeric complexes with racemic mixtures, as described in separation science literature. Its structure makes it suitable for creating peptidomimetics with enhanced metabolic stability, as detailed in bioorganic chemistry studies.
Reviews
Write Your Own Review



![tert-butyl 4-oxohexahydrocyclopenta[c]pyrrole-2(1H)-carboxylate](https://www.chemshuttle.com/media/catalog/product/cache/31dbf0bffbfa69a5826a72cec9a446de/1/5/150205_1.jpg)
![1-methyl-1H,4H,5H,6H-cyclopenta[b]pyrrol-4-one](https://www.chemshuttle.com/media/catalog/product/cache/31dbf0bffbfa69a5826a72cec9a446de/1/9/195465_2.jpg)