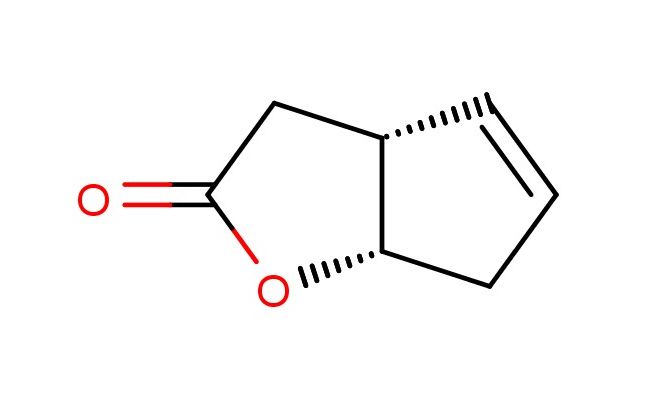
(3aR,6aS)-3,3a,6,6a-tetrahydro-2H-cyclopenta[b]furan-2-one
$400.00
CAS No.: 43119-28-4
Catalog No.: 196176
Purity: 95%
MF: C7H8O2
MW: 124.139
Storage: 2-8 degree Celsius
SMILES: O1[C@@H]2[C@H](CC1=O)C=CC2
Catalog No.: 196176
Purity: 95%
MF: C7H8O2
MW: 124.139
Storage: 2-8 degree Celsius
SMILES: O1[C@@H]2[C@H](CC1=O)C=CC2
For R&D use only. Not for human or animal use.
(3aR,6aS)-3,3a,6,6a-tetrahydro-2H-cyclopenta[b]furan-2-one; CAS No.: 43119-28-4; (3aR,6aS)-3,3a,6,6a-tetrahydro-2H-cyclopenta[b]furan-2-one. PROPERTIES: This compound features a (3aR,6aS)-3,3a,6,6a-tetrahydro-2H-cyclopenta[b]furan-2-one structure with multiple stereocenters and a ketone group. It typically appears as a colorless to pale yellow liquid with a molecular weight of approximately 152.1 g/mol (C7H10O2). The density is around 1.15 g/cm?, and it has a boiling point of approximately 180-185 C at 760 mmHg. It exhibits moderate solubility in common organic solvents like ethyl acetate, dichloromethane, and tetrahydrofuran while being sparingly soluble in water. Proper storage requires a tightly sealed container in a cool, dry place. Safety precautions include wearing appropriate PPE. It is classified as a skin and eye irritant (GHS07) with the hazard statement H315-H319. APPLICATIONS: (3aR,6aS)-3,3a,6,6a-Tetrahydro-2H-cyclopenta[b]furan-2-one serves as a specialized intermediate in pharmaceutical and flavor research. Its (3aR,6aS)-3,3a,6,6a-tetrahydro-2H-cyclopenta[b]furan-2-one structure provides a chiral, cyclic framework suitable for developing enantioselective catalysts and natural product-inspired molecules. In medicinal chemistry, it is used to create bioactive compounds with specific spatial arrangements, particularly in projects targeting protein-protein interactions and enzyme inhibition. The ketone group can undergo nucleophilic addition, reduction to alcohol, or formation of ketals and acetals. This compound also functions as a building block in the synthesis of complex carbohydrates and glycosides. Academic studies employ it as a model compound in Organic Chemistry journals, focusing on asymmetric synthesis and the development of novel chiral architectures based on the (3aR,6aS)-3,3a,6,6a-tetrahydro-2H-cyclopenta[b]furan-2-one scaffold.
Reviews
Write Your Own Review



![tetrahydro-1H-cyclopenta[c]furan-1,3(3aH)-dione](https://www.chemshuttle.com/media/catalog/product/cache/31dbf0bffbfa69a5826a72cec9a446de/1/8/188982_1.jpg)
![[1,1'-Biphenyl]-4-carboxylic acid (3aR,4R,5R,6aS)-hexahydro-2-oxo-4-[(1E)-3-oxo-5-phenyl-1-pentenyl]-2H-cyclopenta[b]furan-5-yl ester](https://www.chemshuttle.com/media/catalog/product/cache/31dbf0bffbfa69a5826a72cec9a446de/w/l/wlz3884_2.jpg)