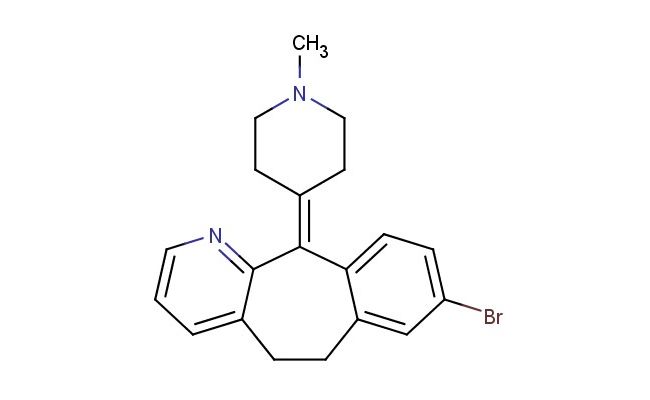
8-bromo-11-(1-methylpiperidin-4-ylidene)-6,11-dihydro-5H-benzo[5,6]cyclohepta[1,2-b]pyridine
$980.00
CAS No.: 130642-57-8
Catalog No.: WLZ0139
Purity: 95%
MF: C20H21BrN2
MW: 369.306
Storage: 2-8 degree Celsius
SMILES: CN1CC/C(CC1)=C2C3=CC=C(Br)C=C3CCC4=CC=CN=C4\2
Catalog No.: WLZ0139
Purity: 95%
MF: C20H21BrN2
MW: 369.306
Storage: 2-8 degree Celsius
SMILES: CN1CC/C(CC1)=C2C3=CC=C(Br)C=C3CCC4=CC=CN=C4\2
For R&D use only. Not for human or animal use.
CAS NO.: 130642-57-8; 8-bromo-11-(1-methylpiperidin-4-ylidene)-6,11-dihydro-5H-benzo[5,6]cyclohepta[1,2-b]pyridine. PROPERTIES: 8-bromo-11-(1-methylpiperidin-4-ylidene)-6,11-dihydro-5H-benzo[5,6]cyclohepta[1,2-b]pyridine appears as a yellow to orange crystalline solid with a characteristic slight pyridine odor. This compound has a molecular formula of C20H19BrN2 and a molecular weight of 369.30 g/mol. It demonstrates low solubility in water but dissolves readily in organic solvents such as dichloromethane, acetone, and dimethyl sulfoxide. The compound should be stored at 2-8 degree Celsius in a tightly sealed container to preserve its stability and prevent degradation. When handling this substance, protective equipment including gloves, eye protection, and lab coats are recommended due to its potential to cause respiratory and skin irritation upon prolonged exposure. The compound is stable under normal laboratory conditions but decomposes upon exposure to strong acids or bases. It is sensitive to light and should be protected from prolonged exposure to UV radiation. The bromine substituent makes it moderately sensitive to nucleophilic attack, and the piperidine nitrogen may gradually react with atmospheric moisture to form hydrates. The melting point typically ranges between 110-115 C, and the compound exhibits a moderate vapor pressure requiring careful handling to prevent volatilization losses. APPLICATIONS: 8-bromo-11-(1-methylpiperidin-4-ylidene)-6,11-dihydro-5H-benzo[5,6]cyclohepta[1,2-b]pyridine serves as a valuable intermediate in the synthesis of complex nitrogen-containing heterocycles. Its unique structural features make it particularly useful in the preparation of serotonin receptor ligands and other central nervous system agents. The bromine substituent at position 8 provides a versatile handle for cross-coupling reactions, enabling the introduction of various aryl and heteroaryl groups to modulate receptor binding profiles. The 1-methylpiperidin-4-ylidene group contributes steric and electronic effects that enhance the compound's ability to interact with specific protein targets. In medicinal chemistry, this compound is frequently used as a building block for the development of atypical antipsychotic agents where the benzocyclohepta[b]pyridine scaffold provides optimal interactions with 5-HT2A and dopamine D2 receptors. The dihydro configuration at positions 6 and 11 allows for controlled reduction pathways that can be exploited in the synthesis of tetracyclic systems with enhanced bioavailability. Researchers in neuropharmacology utilize this compound to develop selective serotonin receptor subtype ligands, enabling more targeted therapeutic interventions for psychiatric disorders. Additionally, the compound's structural framework makes it suitable for photoaffinity labeling studies where the bromine can be replaced with a photoreactive group to capture receptor-prodrug complexes. The unique geometry of the piperidinylidene group facilitates specific hydrogen bonding interactions that are crucial for high-affinity binding to target receptors. In organic chemistry, derivatives of this compound are employed as templates for studying structure-activity relationships in complex polycyclic systems. The rigid cycloheptane ring provides conformational restriction that enhances binding affinity by pre-organizing the molecule into a bioactive conformation. The compound also serves as a precursor for synthesizing fluorescent probes used in neuroscience research to visualize serotonin receptor distributions in brain tissue sections. Furthermore, its structural features make it suitable for developing blood-brain barrier penetrating agents where the lipophilic bromine and piperidine groups facilitate crossing into the central nervous system. The compound's ability to undergo selective reductions and oxidations makes it a versatile intermediate in the synthesis of various tetracyclic and pentacyclic alkaloid-inspired structures with potential therapeutic applications.
Reviews
Write Your Own Review



![7,8-dihydro-5H-cyclohepta[b]pyridine-5,9(6H)-dione](https://www.chemshuttle.com/media/catalog/product/cache/31dbf0bffbfa69a5826a72cec9a446de/1/9/199033_1.jpg)
![5H-benzo[4,5]cyclohepta[1,2-b]pyridin-5-one](https://www.chemshuttle.com/media/catalog/product/cache/31dbf0bffbfa69a5826a72cec9a446de/1/8/180983.jpg)