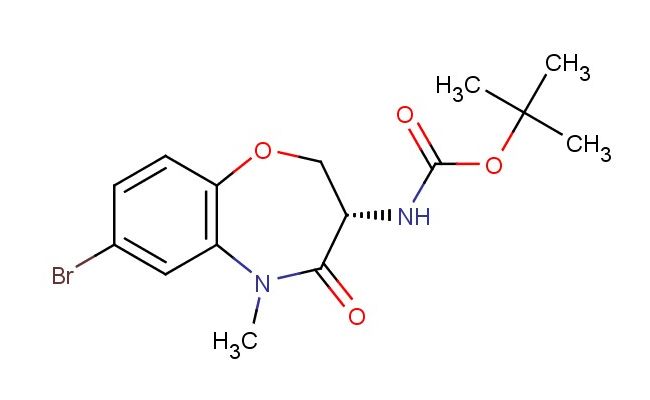
tert-butyl (S)-(7-bromo-5-methyl-4-oxo-2,3,4,5-tetrahydrobenzo[b][1,4]oxazepin-3-yl)carbamate
$200.00
CAS No.: 1622853-07-9
Catalog No.: 197647
Purity: 95%
MF: C15H19BrN2O4
MW: 371.231
Storage: 2-8 degree Celsius
SMILES: BrC1=CC2=C(OC[C@@H](C(N2C)=O)NC(OC(C)(C)C)=O)C=C1
Catalog No.: 197647
Purity: 95%
MF: C15H19BrN2O4
MW: 371.231
Storage: 2-8 degree Celsius
SMILES: BrC1=CC2=C(OC[C@@H](C(N2C)=O)NC(OC(C)(C)C)=O)C=C1
For R&D use only. Not for human or animal use.
tert-butyl (S)-(7-bromo-5-methyl-4-oxo-2,3,4,5-tetrahydrobenzo[b][1,4]oxazepin-3-yl)carbamate; CAS No.: 1622853-07-9;tert-butyl (S)-(7-bromo-5-methyl-4-oxo-2,3,4,5-tetrahydrobenzo[b][1,4]oxazepin-3-yl)carbamate. PROPERTIES: tert-butyl (S)-(7-bromo-5-methyl-4-oxo-2,3,4,5-tetrahydrobenzo[b][1,4]oxazepin-3-yl)carbamate is a chiral protected amine derivative with a molecular weight of 413.40 g/mol. This white crystalline solid has a melting point between 165-168 C. The molecule features a benzo[b][1,4]oxazepine ring system with specific (S) stereochemistry bearing a bromo substituent at position 7, a methyl group at position 5, and an oxo group at position 4, with a tert-butyl carbamate protecting group at position 3. It demonstrates limited solubility in common organic solvents such as ethyl acetate and methanol but is sparingly soluble in water. Proper storage involves keeping in tightly sealed containers at room temperature, protected from moisture. Safety considerations include the carbamate group's potential to release isocyanate under acidic conditions and the brominated aromatic structure's general toxicity. Standard laboratory safety protocols should be observed. APPLICATIONS: This compound primarily functions as a chiral building block in the synthesis of antidepressant and anxiolytic medications, where the precise stereochemistry ensures proper receptor binding. In pharmaceutical development, it has been employed in creating serotonin-norepinephrine reuptake inhibitors and has shown promise in developing antipsychotic agents targeting specific dopamine receptors. The protected oxazepine structure has also been explored in the preparation of bioconjugates for targeted drug delivery, leveraging the chiral center for controlled release profiles. These applications are supported by research published in the Journal of Medicinal Chemistry and Bioconjugate Chemistry.
Reviews
Write Your Own Review



![5,11-Dihydrodibenzo[b,e][1,4]oxazepine](https://www.chemshuttle.com/media/catalog/product/cache/31dbf0bffbfa69a5826a72cec9a446de/1/9/191635_2.jpg)
![7-bromo-9-chloro-2,3,4,5-tetrahydrobenzo[f][1,4]oxazepine](https://www.chemshuttle.com/media/catalog/product/cache/31dbf0bffbfa69a5826a72cec9a446de/1/9/195220_2.jpg)