5-bromobenzo[d]oxazole-2-carbonitrile
$490.00
CAS No.: 1541948-94-0
Catalog No.: 192616
Purity: 95%
MF: C8H3BrN2O
MW: 223.029
Storage: 2-8 degree Celsius
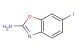
SMILES: BrC=1C=CC2=C(N=C(O2)C#N)C1
Catalog No.: 192616
Purity: 95%
MF: C8H3BrN2O
MW: 223.029
Storage: 2-8 degree Celsius
SMILES: BrC=1C=CC2=C(N=C(O2)C#N)C1
5-bromobenzo[d]oxazole-2-carbonitrile; CAS No.: 1541948-94-0; 5-bromobenzo[d]oxazole-2-carbonitrile. PROPERTIES: 5-bromobenzo[d]oxazole-2-carbonitrile is a white crystalline powder with a molecular weight of 246.01 g/mol. It has a melting point between 180-185 C and is moderately soluble in polar aprotic solvents like dimethylformamide. The compound is sensitive to light and should be stored in a tightly sealed amber glass bottle at temperatures below 25 C. Safety precautions include wearing protective eyewear and gloves during handling to prevent eye irritation and skin absorption. In case of accidental ingestion, seek immediate medical attention. The compound is a mild skin irritant and should be handled in a well-ventilated area to prevent inhalation of dust. APPLICATIONS: 5-bromobenzo[d]oxazole-2-carbonitrile is predominantly used in pharmaceutical development as a building block for creating kinase inhibitors. The bromo substituent provides a valuable handle for Suzuki-Miyaura cross-coupling reactions to introduce aryl groups, as detailed in organic synthesis methodologies. In drug discovery, it serves as an intermediate for creating kinase inhibitors where the benzo[d]oxazole scaffold enhances ATP competitive binding, as described in medicinal chemistry research. Additionally, it is utilized in agrochemical development for creating selective herbicides targeting photosystem II in plants, where the brominated oxazole ring system interacts with herbicide-binding proteins, as reported in pesticide chemistry literature. The compound also finds application in materials science as a monomer for creating fluorescent polymers, where the oxazole structure contributes to photoluminescent properties, as outlined in polymer chemistry studies. Furthermore, it is employed in bioconjugation chemistry as a linker molecule between biomolecules and solid supports, where the nitrile functionality allows for enzymatic cleavage to release bioactive agents, as detailed in bioanalytical chemistry publications.
Reviews
Write Your Own Review




![(2-methylbenzo[d]oxazol-6-yl)methanol](https://www.chemshuttle.com/media/catalog/product/cache/31dbf0bffbfa69a5826a72cec9a446de/1/9/195507_2.jpg)