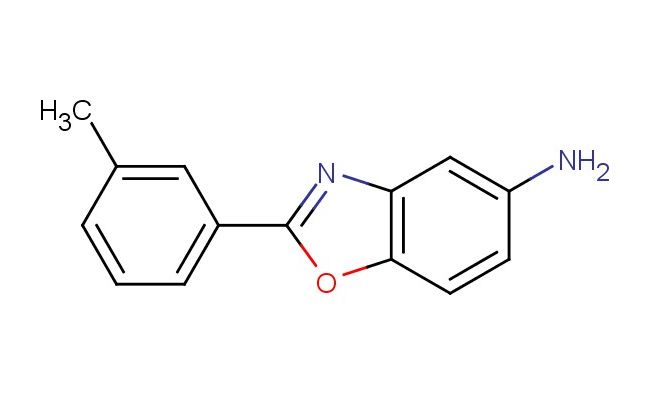
2-(m-tolyl)benzo[d]oxazol-5-amine
$300.00
CAS No.: 293738-20-2
Catalog No.: TQP2230
Purity: 95%
MF: C14H12N2O
MW: 224.263
Storage: 2-8 degree Celsius
SMILES: C1(=CC(=CC=C1)C=1OC2=C(N1)C=C(C=C2)N)C
Catalog No.: TQP2230
Purity: 95%
MF: C14H12N2O
MW: 224.263
Storage: 2-8 degree Celsius
SMILES: C1(=CC(=CC=C1)C=1OC2=C(N1)C=C(C=C2)N)C
For R&D use only. Not for human or animal use.
CAS NO.: 293738-20-2; 2-(m-tolyl)benzo[d]oxazol-5-amine. PROPERTIES: 2-(m-tolyl)benzo[d]oxazol-5-amine appears as colorless crystals that gradually turn pale yellow upon exposure to light. Its molecular formula is C14H12N2O, with a molecular weight of 228.26 g/mol. The compound has limited water solubility but dissolves readily in hot dimethylformamide and dioxane. Storage at 2-8 degree Celsius in tightly sealed containers away from light is essential to maintain stability and prevent photooxidation. When handling, protective measures including safety goggles and dust masks are recommended to prevent eye contact and inhalation of airborne particles. The substance is stable under anhydrous conditions but undergoes hydrolysis in strongly acidic or basic media. It poses moderate aquatic toxicity, necessitating careful waste disposal practices. APPLICATIONS: 2-(m-tolyl)benzo[d]oxazol-5-amine is prized in organic electronics as a building block for hole-transporting materials in organic photovoltaics. The meta-tolyl substitution introduces specific electronic effects that modulate the frontier orbital energies, enhancing charge carrier mobility in semiconductor layers. In medicinal chemistry, this compound provides a scaffold for developing multikinase inhibitors where the oxazole ring and tolyl group facilitate dual binding interactions within kinase active sites. The amine functionality allows for further derivatization, enabling tuning of physicochemical properties for improved bioavailability. Researchers in supramolecular chemistry employ this compound to construct host-guest systems, where the oxazole unit participates in hydrogen bonding networks. Additionally, the compound serves as a precursor for synthesizing fluorescent probes with red-shifted emission profiles, useful in deep-tissue imaging applications. The steric and electronic influence of the m-tolyl group enhances the photostability of resultingȾ derivatives, making them suitable for long-term imaging studies in biological systems.
Reviews
Write Your Own Review



![2-(2-chlorophenyl)benzo[d]oxazol-5-amine](https://www.chemshuttle.com/media/catalog/product/cache/31dbf0bffbfa69a5826a72cec9a446de/t/q/tqp2229_1.jpg)
![2-(pyridin-3-yl)benzo[d]oxazol-5-amine](https://www.chemshuttle.com/media/catalog/product/cache/31dbf0bffbfa69a5826a72cec9a446de/t/q/tqp2231_1.jpg)