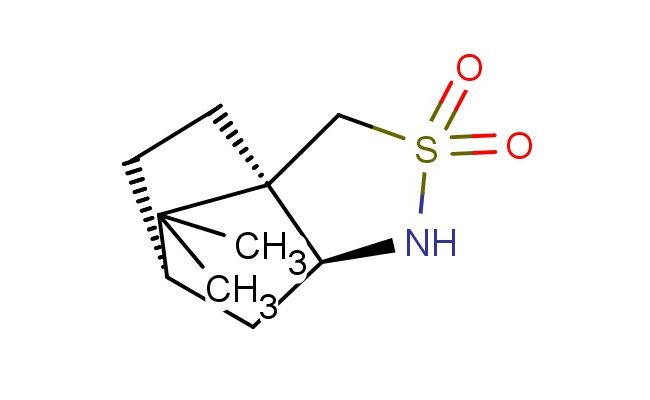
(3aR,6S,7aS)-8,8-dimethylhexahydro-1H-3a,6-methanobenzo[c]isothiazole 2,2-dioxide
$360.00
CAS No.: 108448-77-7
Catalog No.: 196164
Purity: 95%
MF: C10H17NO2S
MW: 215.318
Storage: 2-8 degree Celsius
SMILES: CC1([C@]23[C@@H](NS(C2)(=O)=O)C[C@@H]1CC3)C
Catalog No.: 196164
Purity: 95%
MF: C10H17NO2S
MW: 215.318
Storage: 2-8 degree Celsius
SMILES: CC1([C@]23[C@@H](NS(C2)(=O)=O)C[C@@H]1CC3)C
(3aR,6S,7aS)-8,8-dimethylhexahydro-1H-3a,6-methanobenzo[c]isothiazole 2,2-dioxide; CAS No.: 108448-77-7; (3aR,6S,7aS)-8,8-dimethylhexahydro-1H-3a,6-methanobenzo[c]isothiazole 2,2-dioxide. PROPERTIES: This compound features a complex (3aR,6S,7aS)-8,8-dimethylhexahydro-1H-3a,6-methanobenzo[c]isothiazole 2,2-dioxide structure with multiple stereocenters and a sulfone group. It typically appears as a white to off-white crystalline solid with a molecular weight of approximately 272.4 g/mol (C12H20N2O3S). The melting point ranges between 120-125 C, and it exhibits moderate solubility in polar organic solvents like DMSO and DMF while being sparingly soluble in non-polar solvents. Proper storage requires a tightly sealed container in a cool, dry place. Safety considerations include using appropriate PPE. It is classified as a skin and eye irritant (GHS07) with the hazard statement H315-H319. APPLICATIONS: (3aR,6S,7aS)-8,8-Dimethylhexahydro-1H-3a,6-methanobenzo[c]isothiazole 2,2-dioxide serves as a specialized intermediate in pharmaceutical research. Its (3aR,6S,7aS)-8,8-dimethylhexahydro-1H-3a,6-methanobenzo[c]isothiazole 2,2-dioxide structure provides a rigid, chiral framework suitable for developing enantioselective catalysts and chiral auxiliaries. In medicinal chemistry, it is used to create bioactive molecules with specific spatial arrangements, particularly in projects targeting protein-protein interactions and enzyme inhibition. The sulfone group can undergo reduction to a sulfide or participate in sulfonium ylide formations. This compound also functions as a building block in the synthesis of complex natural product-inspired molecules. Academic research employs it as a model system in Organic Chemistry journals, focusing on asymmetric synthesis and the development of novel chiral architectures based on the (3aR,6S,7aS)-8,8-dimethylhexahydro-1H-3a,6-methanobenzo[c]isothiazole 2,2-dioxide scaffold.
Reviews
Write Your Own Review



![ethyl 2-bromobenzo[d]thiazole-6-carboxylate](https://www.chemshuttle.com/media/catalog/product/cache/31dbf0bffbfa69a5826a72cec9a446de/1/9/196163_2.jpg)
![6-nitrobenzo[d]isothiazol-3(2H)-one 1,1-dioxide](https://www.chemshuttle.com/media/catalog/product/cache/31dbf0bffbfa69a5826a72cec9a446de/1/9/196165_2.jpg)