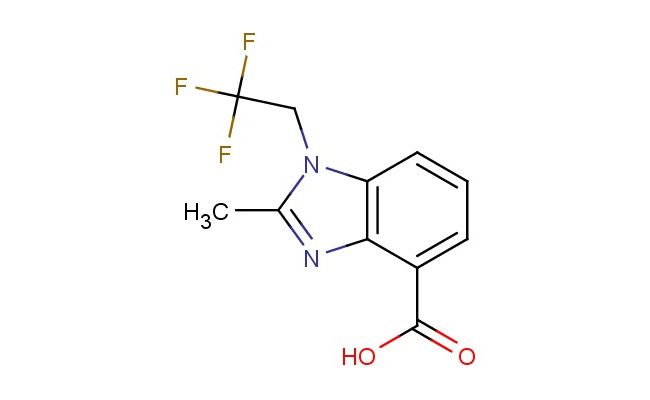
2-methyl-1-(2,2,2-trifluoroethyl)-1H-benzo[d]imidazole-4-carboxylic acid
$400.00
CAS No.: 1553562-87-0
Catalog No.: LT0012
Purity: 95%
MF: C11H9F3N2O2
MW: 258.199
Storage: 2-8 degree Celsius
SMILES: CC1=NC2=C(N1CC(F)(F)F)C=CC=C2C(=O)O
Catalog No.: LT0012
Purity: 95%
MF: C11H9F3N2O2
MW: 258.199
Storage: 2-8 degree Celsius
SMILES: CC1=NC2=C(N1CC(F)(F)F)C=CC=C2C(=O)O
For R&D use only. Not for human or animal use.
CAS NO.: 1553562-87-0;2-methyl-1-(2,2,2-trifluoroethyl)-1H-benzo[d]imidazole-4-carboxylic acid. PROPERTIES: This fluorinated imidazole derivative presents as a white crystalline solid with a molecular weight of approximately 277.1 g/mol. The 2-methyl-1-(2,2,2-trifluoroethyl)-1H-benzo[d]imidazole-4-carboxylic acid combines a trifluoroethyl substituent with a methyl group on a benzimidazole ring. It exhibits limited aqueous solubility but good dissolution in polar aprotic solvents like DMSO and DMF. Stability characterization reveals vulnerability to acid-catalyzed ring-opening and base-promoted hydrolysis of the carboxylic acid group, necessitating storage at 2-8 degree Celsius in sealed glass containers. Handlers should use powder hoods with HEPA filtration and wear cut-resistant gloves during handling. Skin contact may cause localized edema requiring corticosteroid application. Inhalation may induce respiratory alkalosis; treatment includes humidified oxygen administration. Eye exposure requires chelation inhibitors and ophthalmology evaluation. Waste should be hydrolyzed with dilute acid prior to disposal. APPLICATIONS: The 2-methyl-1-(2,2,2-trifluoroethyl)-1H-benzo[d]imidazole-4-carboxylic acid serves as a key intermediate in the synthesis of various pharmaceuticals. Its benzimidazole framework provides opportunities for directed metalation and cross-coupling reactions. Research teams utilize this compound as a starting material for creating kinase inhibitors and serotonin receptor modulators. The trifluoromethyl group enhances metabolic resistance in resulting drug candidates. Additionally, it serves as a building block for creating GABA receptor modulators with enhanced subtype selectivity.
Reviews
Write Your Own Review



![(1H-benzo[d]imidazol-5-yl)(piperazin-1-yl)methanone hydrochloride](https://www.chemshuttle.com/media/catalog/product/cache/31dbf0bffbfa69a5826a72cec9a446de/g/s/gs3394.jpg)
![1-(2,2,2-trifluoroethyl)-1H-benzo[d]imidazole-4-carboxylic acid](https://www.chemshuttle.com/media/catalog/product/cache/31dbf0bffbfa69a5826a72cec9a446de/l/t/lt0015.jpg)