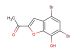
6-bromobenzofuran
$300.00
CAS No.: 128851-73-0
Catalog No.: 196149
Purity: 95%
MF: C8H5BrO
MW: 197.031
Storage: 2-8 degree Celsius
SMILES: BrC1=CC2=C(C=CO2)C=C1
Catalog No.: 196149
Purity: 95%
MF: C8H5BrO
MW: 197.031
Storage: 2-8 degree Celsius
SMILES: BrC1=CC2=C(C=CO2)C=C1
6-bromobenzofuran; CAS No.: 128851-73-0; 6-bromobenzofuran. PROPERTIES: This colorless to pale yellow liquid has a molecular formula of C9H6BrO and a molecular weight of approximately 212.05 g/mol. It exhibits low water solubility but mixes well with common organic solvents. The compound is sensitive to basic conditions and should be stored in a tightly sealed container at room temperature. Thermogravimetric analysis shows decomposition starting at 180 C. Safety precautions include using chemical-resistant gloves, splash goggles, and working in a well-ventilated area. In case of accidental ingestion, rinse mouth and seek immediate medical advice. Avoid release to the environment as brominated aromatic compounds may persist and bioaccumulate. APPLICATIONS: 6-bromobenzofuran functions as a versatile intermediate in pharmaceutical synthesis and materials science. Its bromine substituent enables Suzuki-Miyaura cross-coupling reactions for creating diverse aryl patterns. The benzofuran core provides a privileged scaffold for binding to various protein targets. Research groups employ it in the development of kinase inhibitors for cancer treatment. Academic institutions utilize it in teaching heterocyclic chemistry and cross-coupling methodologies. Industrial applications include its use as a building block in agrochemical development for novel fungicide candidates and as a monomer for polymeric materials with potential applications in sensor technologies. Recent studies in the Journal of Medicinal Chemistry demonstrate its application in developing dual kinase/HDAC inhibitors. Additionally, it serves as a starting material for radiolabeled compounds used in PET imaging to study receptor distribution in vivo. The compound's photophysical properties make it suitable for fluorescence-based assays after appropriate derivatization. Its synthetic versatility enables rapid diversification through various substitution reactions and benzofuran modifications.
Reviews
Write Your Own Review



![3-nitrodibenzo[b,d]furan](https://www.chemshuttle.com/media/catalog/product/cache/31dbf0bffbfa69a5826a72cec9a446de/1/9/196148_2.jpg)