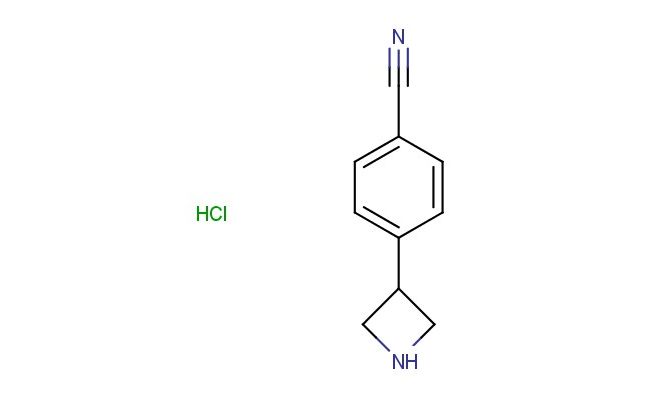
4-(azetidin-3-yl)benzonitrile hydrochloride
$536.00
CAS No.: 94268-29-8
Catalog No.: GS0578
Purity: 95%
MF: C10H11ClN2
MW: 194.665
Storage: 2-8 degree Celsius
SMILES: Cl.N1CC(C1)C1=CC=C(C#N)C=C1
Catalog No.: GS0578
Purity: 95%
MF: C10H11ClN2
MW: 194.665
Storage: 2-8 degree Celsius
SMILES: Cl.N1CC(C1)C1=CC=C(C#N)C=C1
For R&D use only. Not for human or animal use.
CAS NO.: 94268-29-8;4-(azetidin-3-yl)benzonitrile hydrochloride. PROPERTIES: This azetidine-containing nitrile salt presents as a white hygroscopic powder with a molecular weight of approximately 209.6 g/mol (free base). The 4-(azetidin-3-yl)benzonitrile hydrochloride combines a nitrile group with a substituted azetidine ring. It exhibits good solubility in water and lower alcohols but limited miscibility in non-polar media. Stability characterization reveals tendency to form hydrates above 35% relative humidity, necessitating storage at 2-8 degree Celsius in sealed polyethylene containers. Handlers should employ deliquescence-resistant tools and maintain environmental humidity below 30%. Skin contact may cause chemical burns in presence of moisture. Inhalation may induce bronchial hyperreactivity; treatment includes anticholinergic inhalers. Eye exposure requires extended rinsing and possible corticosteroid application. Waste should be neutralized with sodium bicarbonate prior to disposal. APPLICATIONS: The 4-(azetidin-3-yl)benzonitrile hydrochloride serves as a key intermediate in the synthesis of azetidine-containing pharmaceuticals. Its nitrile group provides opportunities for reduction to primary amines or hydrolysis to carboxylic acids. Research teams utilize this compound as a starting material for creating muscarinic receptor modulators and dual serotonin-norepinephrine reuptake inhibitors. The azetidine ring undergoes reductive amination for constructing tertiary amines with defined stereochemistry. Additionally, it serves as a building block for creating kinase inhibitors with enhanced metabolic stability. The compound's unique substitution pattern makes it valuable in the development of GPCR modulators and ion channel inhibitors.
Reviews
Write Your Own Review