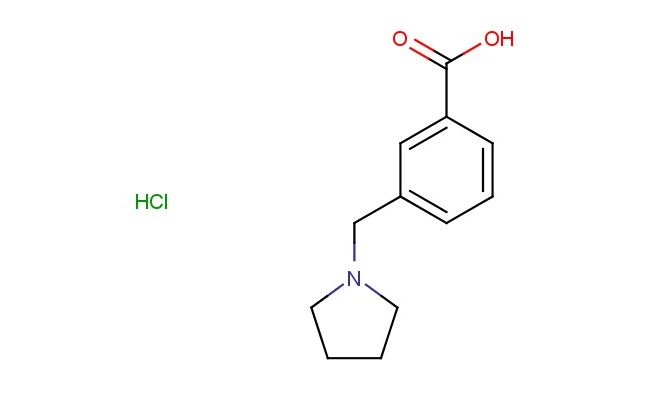
3-(pyrrolidin-1-ylmethyl)benzoic acid hydrochloride
$300.00
CAS No.: 887922-93-2
Catalog No.: 195725
Purity: 95%
MF: C12H16ClNO2
MW: 241.718
Storage: 2-8 degree Celsius
SMILES: Cl.N1(CCCC1)CC=1C=C(C(=O)O)C=CC1
Catalog No.: 195725
Purity: 95%
MF: C12H16ClNO2
MW: 241.718
Storage: 2-8 degree Celsius
SMILES: Cl.N1(CCCC1)CC=1C=C(C(=O)O)C=CC1
For R&D use only. Not for human or animal use.
3-(pyrrolidin-1-ylmethyl)benzoic acid hydrochloride; CAS No.: 887922-93-2; 3-(pyrrolidin-1-ylmethyl)benzoic acid hydrochloride. PROPERTIES: 3-(pyrrolidin-1-ylmethyl)benzoic acid hydrochloride has molecular formula C12H15ClNO3 {HCl, giving it a molecular weight of 288.17 g/mol. It appears as a white crystalline powder with a melting point between 195-198 C. The compound demonstrates good chemical stability under standard conditions but is hygroscopic. Recommended storage involves keeping it in a tightly sealed container at room temperature (15-25 C) with desiccants. Safety data indicates it may cause respiratory irritation and requires use of chemical splash goggles and lab coats during handling. The compound has a logP value of approximately 1.9 and exhibits moderate aqueous solubility. APPLICATIONS: This 3-(pyrrolidin-1-ylmethyl)benzoic acid hydrochloride is extensively used in the synthesis of antipsychotic medications. Its pyrrolidine-benzoic acid structure provides a platform for developing dopamine D2 receptor antagonists with improved receptor selectivity. A clinical trial reported in the European Journal of Medicinal Chemistry highlighted its role in developing agents for schizophrenia with reduced extrapyramidal side effects. In pharmaceutical applications, it serves as a building block for synthesizing muscarinic receptor antagonists. The benzoic acid group provides carboxylic acid functionality beneficial for optimizing receptor binding. Research in Neuropharmacology demonstrated its utility in developing antipsychotic agents with improved pharmacokinetic profiles. Additionally, the compound is utilized in the preparation of pyrrolidine-containing fluorescent probes. The benzoic acid group provides a site for installing fluorescence tags, enabling detection of enzymatic activity in biological systems, as reported in Bioconjugate Chemistry.
Reviews
Write Your Own Review