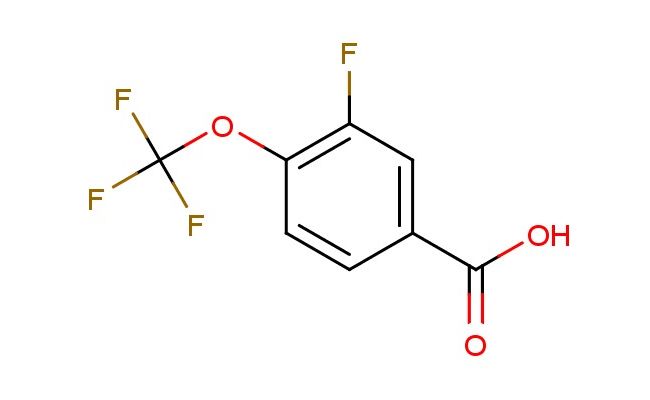
3-fluoro-4-(trifluoromethoxy)benzoic acid
$250.00
CAS No.: 886498-89-1
Catalog No.: 200382
Purity: 95%
MF: C8H4F4O3
MW: 224.109
Storage: 2-8 degree Celsius
SMILES: FC=1C=C(C(=O)O)C=CC1OC(F)(F)F
Catalog No.: 200382
Purity: 95%
MF: C8H4F4O3
MW: 224.109
Storage: 2-8 degree Celsius
SMILES: FC=1C=C(C(=O)O)C=CC1OC(F)(F)F
CAS NO.: 886498-89-1;3-fluoro-4-(trifluoromethoxy)benzoic acid. PROPERTIES: This polyfluorinated aromatic acid appears as colorless prisms with molecular weight approximately 231.0 g/mol, combining fluorobenzene core with trifluoromethoxy and carboxylic acid groups. The 3-fluoro-4-(trifluoromethoxy)benzoic acid exhibits limited aqueous solubility until pH < 2 but good dissolution in acetone and ethyl acetate. Stability characterization reveals susceptibility to thermal decarboxylation above 180 C and acid-catalyzed trifluoromethyl migration, necessitating storage at 2-8 degree Celsius in glass-lined containers. Handlers should employ PTFE-lined transfer devices and avoid using aluminum foil which may promote unwanted redox reactions. Skin contact may cause localized vasoconstriction requiring warming of affected areas. Inhalation of dust may induce hypocalcemia; treatment includes calcium gluconate administration. Eye exposure requires chelation inhibitors and immediate ophthalmology consultation. Waste should be incinerated at >1200 C to prevent dioxin formation. APPLICATIONS: The 3-fluoro-4-(trifluoromethoxy)benzoic acid serves as a key intermediate in the preparation of tyrosine kinase inhibitors and matrix metalloproteinase inhibitors. Its trifluoromethoxy group provides enhanced lipophilicity and metabolic resistance compared to parent fluoro analogs. The compound functions as a building block for creating benzamide antipsychotics with improved blood-brain barrier penetration. Additionally, it undergoes Ullmann reaction for constructing biaryl systems. Research teams employ it as a starting material for creating fluorescent probes with red-shifted emission. In materials science, its electron-deficient aromatic system enables creation of high-performance liquid crystal displays with improved response times.
Reviews
Write Your Own Review