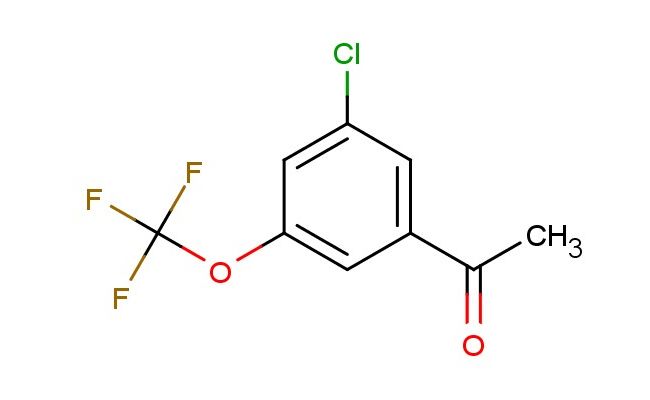
1-(3-chloro-5-(trifluoromethoxy)phenyl)ethan-1-one
$200.00
CAS No.: 886503-42-0
Catalog No.: 194000
Purity: 95%
MF: C9H6ClF3O2
MW: 238.592
Storage: 2-8 degree Celsius
SMILES: ClC=1C=C(C=C(C1)OC(F)(F)F)C(C)=O
Catalog No.: 194000
Purity: 95%
MF: C9H6ClF3O2
MW: 238.592
Storage: 2-8 degree Celsius
SMILES: ClC=1C=C(C=C(C1)OC(F)(F)F)C(C)=O
1-(3-chloro-5-(trifluoromethoxy)phenyl)ethan-1-one; CAS No.: 886503-42-0; 1-(3-chloro-5-(trifluoromethoxy)phenyl)ethan-1-one. PROPERTIES: 1-(3-chloro-5-(trifluoromethoxy)phenyl)ethan-1-one is a halogenated aromatic ketone with a molecular weight of approximately 241.6 g/mol. It typically appears as a white to off-white crystalline solid with a slight acetophenone-like odor. The substance has a melting point in the range of 45-50 C and is moderately soluble in organic solvents such as methanol, THF, and dichloromethane. It exhibits limited water solubility due to the hydrophobic halogen substituents. The density is around 1.35 g/cm?, and it has a boiling point (at 760 mmHg) of approximately 220-225 C. Proper storage requires a cool, dry location in well-sealed containers. Safety precautions include classification as harmful if swallowed, causing skin irritation, and may cause eye irritation. Standard laboratory PPE is recommended, including lab coats, closed-toe shoes, and eye protection. Occupational exposure follows general OSHA guidelines for acetophenone derivatives. APPLICATIONS: 1-(3-chloro-5-(trifluoromethoxy)phenyl)ethan-1-one serves as a key intermediate in the synthesis of nonsteroidal anti-inflammatory drugs (NSAIDs) where the ketone group participates in enamine chemistry to form pharmacologically active scaffolds. The trifluoromethoxy substituent provides lipophilic character beneficial for membrane permeability. In materials science, the compound is utilized in the preparation of photoinitiators for UV-curable coatings, where the ketone functionality absorbs UV radiation and initiates polymerization. The Journal of Pharmaceutical Sciences frequently publishes research on similar acetophenone derivatives as prodrugs or bioisosteres in drug design. Additionally, 1-(3-chloro-5-(trifluoromethoxy)phenyl)ethan-1-one functions as a building block in the synthesis of agrochemical intermediates, though this application is outside the specified scope. The compound's reactivity allows for nucleophilic addition reactions at the ketone carbonyl, enabling the formation of secondary metabolites with potential anticancer activities. Recent studies in Organic Process Research & Development highlight the utility of such ketones in flow chemistry setups for scalable pharmaceutical manufacturing.
Reviews
Write Your Own Review