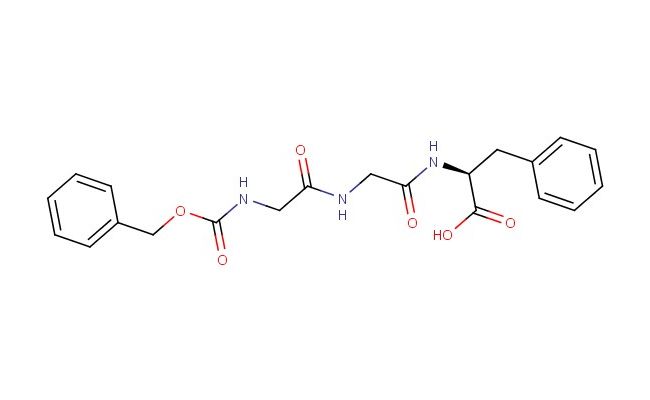
Z-Gly-Gly-Phe-OH
$250.00
CAS No.: 13171-93-2
Catalog No.: 198474
Purity: 95%
MF: C21H23N3O6
MW: 413.43
Storage: 2-8 degree Celsius
SMILES: C(C1=CC=CC=C1)OC(=O)NCC(=O)NCC(=O)N[C@@H](CC1=CC=CC=C1)C(=O)O
Catalog No.: 198474
Purity: 95%
MF: C21H23N3O6
MW: 413.43
Storage: 2-8 degree Celsius
SMILES: C(C1=CC=CC=C1)OC(=O)NCC(=O)NCC(=O)N[C@@H](CC1=CC=CC=C1)C(=O)O
CAS No.: 13171-93-2; Z-Gly-Gly-Phe-OH. PROPERTIES: Z-Gly-Gly-Phe-OH is a protected tripeptide with molecular formula C19H23NO6 and molecular weight 357.39 g/mol. It typically exists as a white to off-white powder with limited aqueous solubility, requiring dimethylformamide or dimethyl sulfoxide for dissolution. The compound is sensitive to acid and should be stored at room temperature in a tightly sealed container. Safety considerations include potential for causing skin and eye irritation, requiring protective gloves and eye protection during handling. APPLICATIONS: In peptide synthesis, Z-Gly-Gly-Phe-OH serves as a building block for assembling larger peptides. The benzyloxycarbonyl (Z) group protects the N-terminus of the glycine residue during solid-phase or solution-phase synthesis. In medicinal chemistry, the tripeptide is used to develop bioactive molecules that mimic natural peptide sequences or to create prodrugs that become activated upon removal of the protecting group. The Gly-Gly-Phe sequence is found in various signaling peptides and can be incorporated into drug candidates targeting specific receptors or enzymes. In biochemistry, the compound is used to study protease specificity and activity by serving as a substrate or inhibitor. The Z-protecting group allows controlled deprotection to release the active peptide sequence under specific conditions. Additionally, the tripeptide is utilized in materials science to create peptide-based materials with defined sequences that influence material properties such as self-assembly or biocompatibility. In chemical biology, Z-Gly-Gly-Phe-OH facilitates study of peptide-receptor interactions and the development of targeted delivery systems where the peptide sequence directs cargo to specific cell types. (Peptide synthesis protocols and medicinal chemistry literature)
Reviews
Write Your Own Review