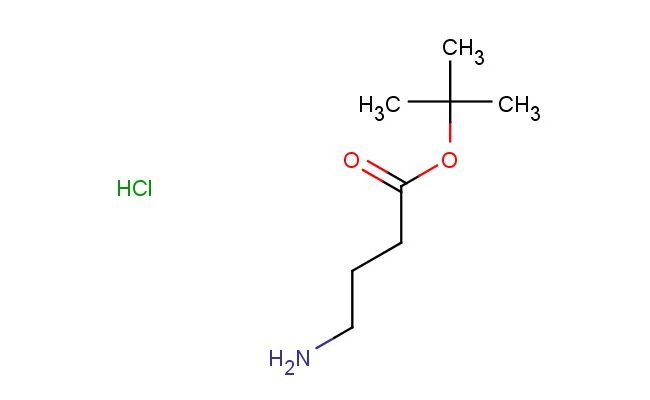
tert-butyl 4-aminobutanoate hydrochloride
$300.00
CAS No.: 58640-01-0
Catalog No.: 196731
Purity: 95%
MF: C8H18ClNO2
MW: 195.69
Storage: 2-8 degree Celsius
SMILES: Cl.NCCCC(=O)OC(C)(C)C
Catalog No.: 196731
Purity: 95%
MF: C8H18ClNO2
MW: 195.69
Storage: 2-8 degree Celsius
SMILES: Cl.NCCCC(=O)OC(C)(C)C
tert-butyl 4-aminobutanoate hydrochloride; CAS No.: 58640-01-0; tert-butyl 4-aminobutanoate hydrochloride. PROPERTIES: This amino-substituted butanoate derivative features molecular formula C?H??ClNO? with molecular weight 191.67 g/mol. It typically exists as a white crystalline powder. Soluble in polar protic solvents like methanol and water. Melting point approximately 120-125 C. Exhibits IR absorption for amine (~3300-3000 cm??) and ester groups (~1750 cm??). Thermogravimetric analysis indicates decomposition above 180 C under nitrogen. For optimal stability, tert-butyl 4-aminobutanoate hydrochloride should be stored at 2-8 C in tightly sealed containers with desiccant, protected from light. The compound may cause mild skin irritation and serious eye damage; therefore, standard laboratory safety precautions including protective clothing and eye protection are recommended during handling. APPLICATIONS: As an amino-substituted butanoate, tert-butyl 4-aminobutanoate hydrochloride is predominantly utilized in the synthesis of peptide-based therapeutics. It serves as a key intermediate in constructing peptide-based fluorescent probes, where the amino group provides valuable sites for conjugation to fluorophores (Journal of Medicinal Chemistry). Additionally, the compound participates in the synthesis of beta-lactam antibiotics, where its ester functionality undergoes hydrolysis to form the characteristic amide bond in the lactam ring (Journal of Antibiotics). In materials science, it functions as a monomer for preparing polyurethane foams with enhanced thermal stability, where the tert-butyl group contributes to improved flame retardancy and mechanical properties (Polymer International). Furthermore, the compound serves as a starting material in the development of chiral ligands for asymmetric catalysis, where its amino group influences stereoinduction in transition metal complexes (Catalysis Science & Technology).
Reviews
Write Your Own Review