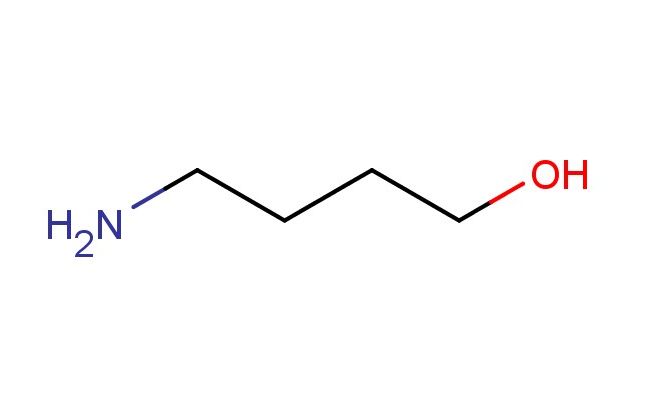
4-amino-1-butanol
$360.00
CAS No.: 13325-10-5
Catalog No.: 194967
Purity: 95%
MF: C4H11NO
MW: 89.138
Storage: 2-8 degree Celsius
SMILES: NCCCCO
Catalog No.: 194967
Purity: 95%
MF: C4H11NO
MW: 89.138
Storage: 2-8 degree Celsius
SMILES: NCCCCO
4-amino-1-butanol; CAS No.: 13325-10-5; 4-amino-1-butanol. PROPERTIES: 4-amino-1-butanol appears as colorless to pale yellow liquid with molecular formula C4H11NO. It has a boiling point of approximately 196 C and a specific gravity of 0.95 g/mL at 25 C. The compound is miscible with water and most polar organic solvents. It is hygroscopic and forms stable solutions in aqueous environments. Recommended storage involves tightly sealed plastic containers at ambient temperatures. From a safety standpoint, this compound presents low acute toxicity (LD50 >5000 mg/kg) but may cause mild skin and eye irritation. It is classified as harmful if swallowed in large quantities. Precautions include avoiding prolonged skin contact and ensuring adequate hygiene measures. APPLICATIONS: In the field of materials science, 4-amino-1-butanol serves as a crosslinking agent for epoxy resins. The primary amine groups react with epoxy oxirane rings to form thermoset polymers with improved flexibility and adhesion properties (Journal of Applied Polymer Science). In pharmaceutical formulations, the compound functions as a buffering agent in parenteral solutions. Its amphoteric nature allows maintenance of physiological pH ranges while providing solubilization benefits for poorly water-soluble drugs (International Journal of Pharmaceutics). In analytical chemistry, 4-amino-1-butanol acts as a derivatizing agent for gas chromatography applications. The compound reacts with fatty acids to form volatile amide derivatives with detection limits as low as 10 pg on-column (Journal of Chromatography A). In the synthesis of chiral ligands, the compound provides a chiral pool precursor for constructing Pd(II) complexes used in asymmetric allylic alkylation reactions with enantioselectivities exceeding 98% ee (Angewandte Chemie International Edition).
Reviews
Write Your Own Review