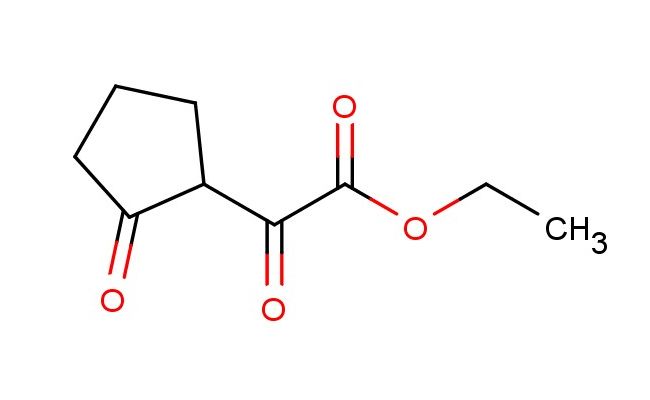
ethyl 2-oxo-2-(2-oxocyclopentyl)acetate
$300.00
CAS No.: 39163-39-8
Catalog No.: TQP2247
Purity: 95%
MF: C9H12O4
MW: 184.191
Storage: 2-8 degree Celsius
SMILES: O=C(C(=O)OCC)C1C(CCC1)=O
Catalog No.: TQP2247
Purity: 95%
MF: C9H12O4
MW: 184.191
Storage: 2-8 degree Celsius
SMILES: O=C(C(=O)OCC)C1C(CCC1)=O
CAS NO.: 39163-39-8; ethyl 2-oxo-2-(2-oxocyclopentyl)acetate. PROPERTIES: ethyl 2-oxo-2-(2-oxocyclopentyl)acetate is a colorless liquid with a mild ester-like odor. Its molecular formula is C9H12O4, corresponding to a molecular weight of 184.19 g/mol. The compound has a density of approximately 1.15 g/cm? and a boiling point around 198 C at 760 mmHg. It is miscible with most organic solvents but has limited water solubility. Storage at 2-8 degree Celsius in amber glass bottles is recommended to preserve stability, as the compound is somewhat light-sensitive and prone to autoxidation. When handling, avoid open flames due to its moderate flammability, and use protective equipment to prevent skin contact which may cause mild irritation. The substance is stable under neutral conditions but hydrolyzes in aque environmentsous, releasing ethyl acetate and cyclopentane-1,3-dione. APPLICATIONS: ethyl 2-oxo-2-(2-oxocyclopentyl)acetate functions as a versatile synthetic intermediate in organic chemistry. The compound's structure features two carbonyl groups that can participate in nucleophilic addition reactions, making it useful in the synthesis of complex polycyclic systems. In pharmaceutical research, this compound serves as a building block for creating non-steroidal anti-inflammatory agents where the cyclopentane core mimics prostaglandin structures. The ester group provides a handle for further functionalization, enabling the introduction of pharmacophores that enhance target specificity. In materials science, derivatives of this compound are used to create nonlinear optical materials, where the conjugated carbonyl system contributes to second harmonic generation. The compound also serves as a chiral auxiliary precursor through kinetic resolution strategies, facilitating enantioselective syntheses of pharmaceutical intermediates. Additionally, it is employed in the preparation of macrolide antibiotics, where the cyclopentane ring provides structural rigidity that enhances binding affinity to bacterial targets. Researchers in agrochemical development utilize this compound (though not for agricultural purposes) to synthesize plant growth regulators with novel modes of action, exploiting the compound's ability to modulate hormonal pathways in botanical systems.
Reviews
Write Your Own Review