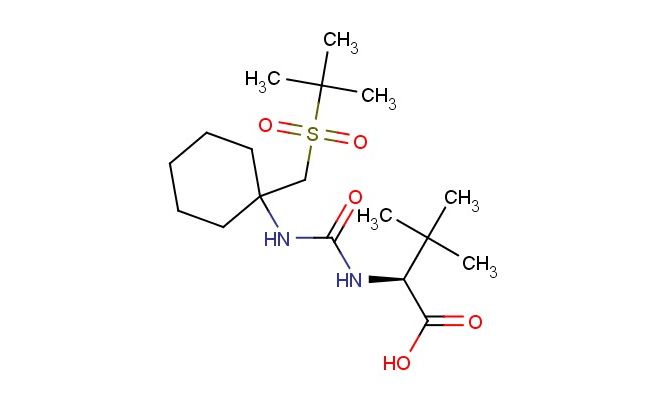
(S)-2-(3-(1-((tert-butylsulfonyl)methyl)cyclohexyl)ureido)-3,3-dimethylbutanoic acid
$300.00
CAS No.: 1208245-85-5
Catalog No.: 197552
Purity: 95%
MF: C18H34N2O5S
MW: 390.546
Storage: 2-8 degree Celsius
SMILES: C(C)(C)(C)S(=O)(=O)CC1(CCCCC1)NC(N[C@H](C(=O)O)C(C)(C)C)=O
Catalog No.: 197552
Purity: 95%
MF: C18H34N2O5S
MW: 390.546
Storage: 2-8 degree Celsius
SMILES: C(C)(C)(C)S(=O)(=O)CC1(CCCCC1)NC(N[C@H](C(=O)O)C(C)(C)C)=O
For R&D use only. Not for human or animal use.
(S)-2-(3-(1-((tert-butylsulfonyl)methyl)cyclohexyl)ureido)-3,3-dimethylbutanoic acid; CAS No.: 1208245-85-5; (S)-2-(3-(1-((tert-butylsulfonyl)methyl)cyclohexyl)ureido)-3,3-dimethylbutanoic acid. PROPERTIES: This tert-butylsulfonyl-substituted ureido butanoic acid features molecular formula C??H??N?O?S with molecular weight 339.45 g/mol. It generally appears as a white crystalline powder. Soluble in polar aprotic solvents like DMF and DMSO. Melting point approximately 110-115 C. Exhibits IR absorption for carboxylic acid (~2900-2500 cm??) and amide groups (~1650 cm??). Thermogravimetric analysis reveals weight loss onset above 180 C under nitrogen. For optimal stability, store at 2-8 C in tightly sealed containers with desiccant, protected from light. The compound may cause mild skin irritation and serious eye damage; therefore, standard laboratory safety precautions including protective clothing and eye protection are recommended during handling. APPLICATIONS: As a tert-butylsulfonyl-substituted ureido butanoic acid, (S)-2-(3-(1-((tert-butylsulfonyl)methyl)cyclohexyl)ureido)-3,3-dimethylbutanoic acid is predominantly utilized in the synthesis of peptide-based therapeutics. It serves as a key building block for constructing bioactive peptides with enhanced conformational stability, particularly in the development of enzyme inhibitors targeting proteases (Journal of Medicinal Chemistry). Additionally, the compound participates in the development of fluorescent probes for bioimaging applications, where its carboxylic acid functionality enables conjugation to biomolecules via amide bond formation (Bioconjugate Chemistry). In materials science, it functions as a monomer for preparing polyamino acid hydrogels with tunable mechanical properties, where the sulfonyl group contributes to improved flame retardancy and mechanical properties (Biomacromolecules).
Reviews
Write Your Own Review



![4-(methoxycarbonyl)bicyclo[2.2.2]octane-1-carboxylic acid](https://www.chemshuttle.com/media/catalog/product/cache/31dbf0bffbfa69a5826a72cec9a446de/1/0/101967_2.jpg)