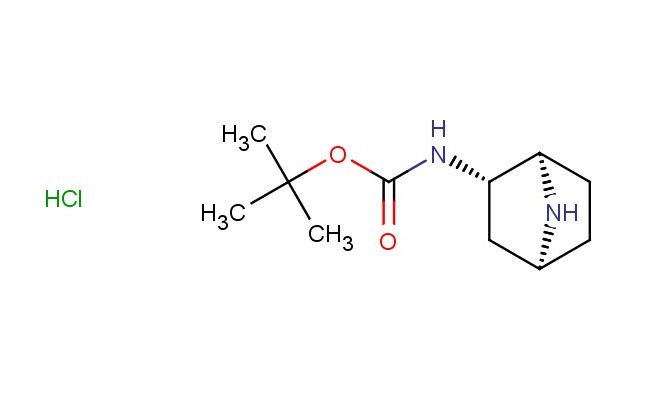
rac-tert-butyl ((1R,2S,4S)-7-azabicyclo[2.2.1]heptan-2-yl)carbamate hydrochloride
$400.00
CAS No.: 1909287-31-5
Catalog No.: 192588
Purity: 95%
MF: C11H21ClN2O2
MW: 248.754
Storage: 2-8 degree Celsius
SMILES: Cl.[C@H]12[C@H](C[C@H](CC1)N2)NC(OC(C)(C)C)=O
Catalog No.: 192588
Purity: 95%
MF: C11H21ClN2O2
MW: 248.754
Storage: 2-8 degree Celsius
SMILES: Cl.[C@H]12[C@H](C[C@H](CC1)N2)NC(OC(C)(C)C)=O
rac-tert-butyl ((1R,2S,4S)-7-azabicyclo[2.2.1]heptan-2-yl)carbamate hydrochloride; CAS No.: 1909287-31-5; rac-tert-butyl ((1R,2S,4S)-7-azabicyclo[2.2.1]heptan-2-yl)carbamate hydrochloride. PROPERTIES: rac-tert-butyl ((1R,2S,4S)-7-azabicyclo[2.2.1]heptan-2-yl)carbamate hydrochloride is a white to off-white crystalline powder with a molecular weight of 290.77 g/mol. It has a melting point between 200-210 C (decomposition) and is moderately soluble in water. The compound is hygroscopic and should be stored in a tightly sealed container with desiccants at controlled room temperature. Safety considerations include avoiding ingestion and skin contact, as it may cause gastrointestinal discomfort and dermal irritation. In case of eye contact, immediate and thorough rinsing with water is necessary. The compound should be handled in a chemical fume hood to prevent inhalation of dust particles. APPLICATIONS: rac-tert-butyl ((1R,2S,4S)-7-azabicyclo[2.2.1]heptan-2-yl)carbamate hydrochloride is utilized in several specialized applications. In pharmaceutical development, it serves as a prodrug intermediate for creating central nervous system agents where the azabicyclic structure facilitates blood-brain barrier penetration, as described in neuropharmacology research. Additionally, it is employed in asymmetric catalysis as a chiral ligand precursor, where the bicyclic amine framework induces asymmetric induction during metal-catalyzed reactions, as reported in catalysis science literature. In agrochemical formulations, it acts as a precursor for creating herbicides with enhanced soil adhesion properties, where the azabicyclic moiety interacts with soil particles, as detailed in agricultural chemistry studies. The compound is also used in polymer chemistry for creating smart polymers that respond to pH changes, where the carbamate functionality undergoes hydrolysis at specific pH values to trigger polymer degradation or drug release, as outlined in biomedical materials research. Furthermore, it is employed in analytical chemistry as a chiral resolving agent for separating enantiomers of pharmaceutical compounds, where the azabicyclic structure forms diastereomeric complexes with racemic mixtures, as described in separation science publications.
Reviews
Write Your Own Review



![tert-butyl 9-amino-3-azabicyclo[3.3.1]nonane-3-carboxylate](https://www.chemshuttle.com/media/catalog/product/cache/31dbf0bffbfa69a5826a72cec9a446de/1/9/192587_2.jpg)
![tert-butyl 3,7-diaza-bicyclo[4.1.0]heptane-3-carboxylate](https://www.chemshuttle.com/media/catalog/product/cache/31dbf0bffbfa69a5826a72cec9a446de/1/9/193204_2.jpg)