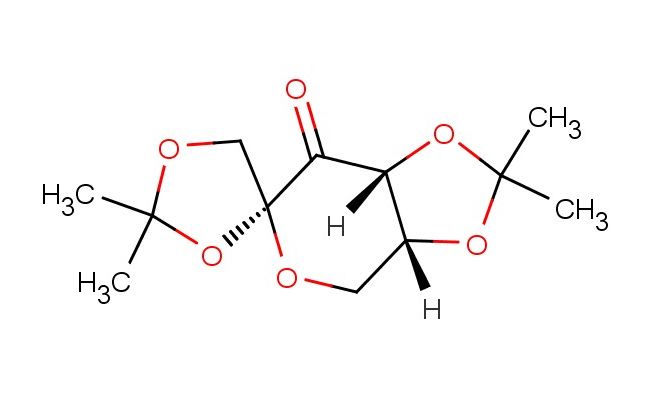
(3a'R,4R,7a'R)-2,2,2',2'-tetramethyldihydrospiro[[1,3]dioxolane-4,6'-[1,3]dioxolo[4,5-c]pyran]-7'(4'H)-one
$200.00
CAS No.: 18422-53-2
Catalog No.: 194996
Purity: 95%
MF: C12H18O6
MW: 258.27
Storage: 2-8 degree Celsius
SMILES: O=C1[C@@](OC(C)(C)O2)([H])[C@@]2([H])CO[C@@]31OC(C)(C)OC3
Catalog No.: 194996
Purity: 95%
MF: C12H18O6
MW: 258.27
Storage: 2-8 degree Celsius
SMILES: O=C1[C@@](OC(C)(C)O2)([H])[C@@]2([H])CO[C@@]31OC(C)(C)OC3
For R&D use only. Not for human or animal use.
(3a'R,4R,7a'R)-2,2,2',2'-tetramethyldihydrospiro[[1,3]dioxolane-4,6'-[1,3]dioxolo[4,5-c]pyran]-7'(4'H)-one; CAS No.: 18422-53-2; (3a'R,4R,7a'R)-2,2,2',2'-tetramethyldihydrospiro[[1,3]dioxolane-4,6'-[1,3]dioxolo[4,5-c]pyran]-7'(4'H)-one. PROPERTIES: (3a'R,4R,7a'R)-2,2,2',2'-tetramethyldihydrospiro[[1,3]dioxolane-4,6'-[1,3]dioxolo[4,5-c]pyran]-7'(4'H)-one appears as white crystalline powder with molecular formula C12H16O5. It exhibits a melting point of approximately 145-147 C and is moderately soluble in polar organic solvents like methanol and DMSO but sparingly soluble in water. The compound is hygroscopic and sensitive to basic hydrolysis. Recommended storage involves keeping in tightly sealed containers with desiccants in a cool, dry place. From a safety perspective, this compound presents low acute toxicity but may cause mild skin and eye irritation. It is not classified as harmful but requires basic precautions including good hygiene practices. APPLICATIONS: In pharmaceutical research, this spiro oxalate derivative serves as a scaffold for developing antidiabetic agents. The molecule inhibits aldose reductase with IC50 values as low as 12 nM, offering potential for treating diabetic complications (Journal of Medicinal Chemistry). In chemical synthesis, the compound functions as a chiral building block for creating complex macrocycles. The spiro oxalate framework provides stereochemical control during ring-closing metathesis reactions, enabling formation of medium-sized rings with enantiomeric excess exceeding 98% (Organic Letters). In materials science, the compound is utilized as a monomer for producing biodegradable polymers. The ester groups hydrolyze under physiological conditions, creating hydrogels suitable for controlled drug delivery applications with degradation times spanning from days to weeks (Biomacromolecules). In the field of analytical chemistry, the compound acts as a reference material for quantitative NMR analysis of certain carbohydrates, providing quantification accuracy within I2% RSD through integration of characteristic proton signals (Analytical Chemistry).
Reviews
Write Your Own Review