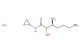
3,3-dimethyl-1-oxa-6-azaspiro[3.3]heptane 2,2,2-trifluoroacetate
$500.00
CAS No.: 1254036-08-2
Catalog No.: 197681
Purity: 95%
MF: C9H14F3NO3
MW: 241.209
Storage: 2-8 degree Celsius
SMILES: FC(C(=O)O)(F)F.CC1(COC12CNC2)C
Catalog No.: 197681
Purity: 95%
MF: C9H14F3NO3
MW: 241.209
Storage: 2-8 degree Celsius
SMILES: FC(C(=O)O)(F)F.CC1(COC12CNC2)C
For R&D use only. Not for human or animal use.
3,3-dimethyl-1-oxa-6-azaspiro[3.3]heptane 2,2,2-trifluoroacetate; CAS No.: 1254036-08-2;3,3-dimethyl-1-oxa-6-azaspiro[3.3]heptane 2,2,2-trifluoroacetate. PROPERTIES: 3,3-dimethyl-1-oxa-6-azaspiro[3.3]heptane 2,2,2-trifluoroacetate is a spirocyclic compound with a molecular weight of 231.27 g/mol. This colorless liquid has a boiling point around 120-122 C at 760 mmHg. The molecule features a spiro[3.3]heptane ring system with an oxaza substitution at position 1 and an azasubstitution at position 6, bearing dimethyl groups at position 3 and a trifluoroacetate protecting group. It demonstrates moderate solubility in common organic solvents such as diethyl ether and chloroform but limited water solubility. Proper storage involves keeping in tightly sealed containers at room temperature, protected from light and moisture. Safety considerations include the trifluoroacetate group's potential to release toxic fumes when heated and the spirocyclic structure's general toxicity. Proper protective equipment should be utilized during handling. APPLICATIONS: This compound primarily serves as a synthetic intermediate in the production of pharmaceuticals and specialty chemicals, where the spirocyclic scaffold provides structural diversity for receptor interactions. In medicinal chemistry, it has been employed in developing antiviral agents targeting RNA viruses and has shown utility in creating kinase inhibitors for cancer therapy. The spirocyclic oxazaspiroheptane structure has also been explored in materials science for developing chiral ligands in asymmetric catalysis, leveraging the dimethyl groups' steric effects. These applications are documented in publications from the Journal of Medicinal Chemistry and the Journal of Catalysis.
Reviews
Write Your Own Review



![tert-butyl 7-oxo-2-azaspiro[4.4]nonane-2-carboxylate](https://www.chemshuttle.com/media/catalog/product/cache/31dbf0bffbfa69a5826a72cec9a446de/1/9/197683_2.jpg)