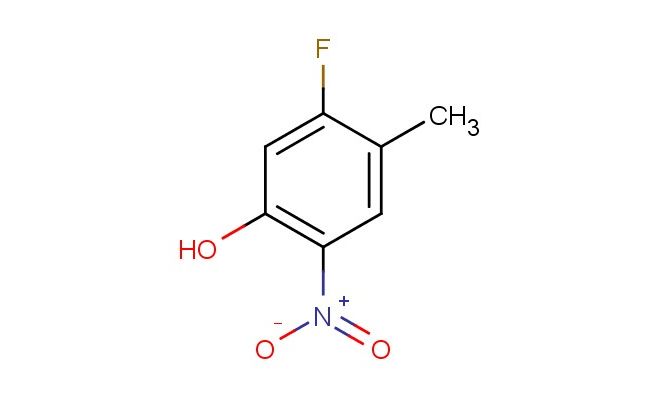
5-fluoro-4-methyl-2-nitrophenol
$200.00
CAS No.: 83341-28-0
Catalog No.: WLZ1785
Purity: 95%
MF: C7H6FNO3
MW: 171.127
Storage: 2-8 degree Celsius
SMILES: FC=1C(=CC(=C(C1)O)[N+](=O)[O-])C
Catalog No.: WLZ1785
Purity: 95%
MF: C7H6FNO3
MW: 171.127
Storage: 2-8 degree Celsius
SMILES: FC=1C(=CC(=C(C1)O)[N+](=O)[O-])C
CAS NO.: 83341-28-0; 5-fluoro-4-methyl-2-nitrophenol. PROPERTIES: This fluoromethylated aromatic compound features a fluorine atom, a methyl group, and a nitro group on a phenol ring, creating a molecule with potential applications in organic synthesis and pharmaceutical research. The 5-fluoro-4-methyl-2-nitrophenol typically appears as a white crystalline powder with moderate aqueous solubility. Its molecular structure includes electron-withdrawing nitro and fluorine groups and an electron-donating methyl group that influence the electronic properties of the aromatic system. For optimal stability, this compound should be stored at 2-8 degree Celsius in a tightly sealed container away from moisture and direct light. When handling, chemists should wear appropriate personal protective equipment including nitrile gloves and safety goggles. This compound is hygroscopic and may form salts upon exposure to atmospheric moisture. In case of accidental ingestion, rinse mouth thoroughly and seek medical attention. APPLICATIONS: The 5-fluoro-4-methyl-2-nitrophenol serves as a valuable intermediate in the synthesis of pharmaceuticals, particularly those targeting enzyme inhibitors and receptor modulators. The nitro group provides a bioisosteric replacement that can enhance metabolic stability and target binding affinity. In medicinal chemistry, this compound functions as a building block for developing antiviral and anticancer agents where the phenolic hydroxy group contributes to target engagement. Additionally, the molecule finds utility in chemical biology studies where its unique scaffold can be used to probe enzyme inhibition mechanisms and cellular signaling pathways. Researchers utilizing this compound benefit from its defined electronic properties and functional group arrangement, advancing investigations into novel therapeutic approaches for various disease conditions.
Reviews
Write Your Own Review