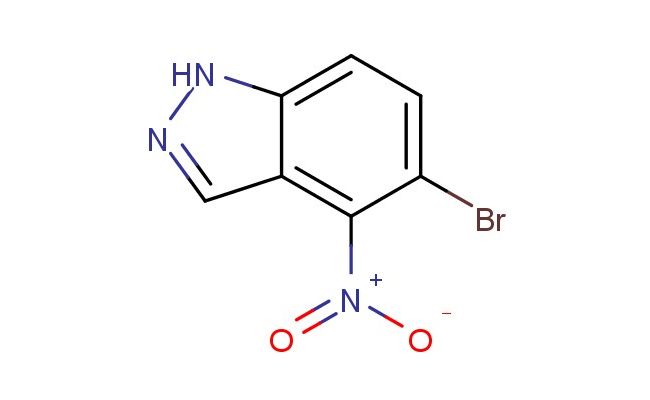
5-bromo-4-nitro-1H-indazole
$250.00
CAS No.: 2133005-85-1
Catalog No.: TQP0943
Purity: 95%
MF: C7H4BrN3O2
MW: 242.032
Storage: 2-8 degree Celsius
SMILES: BrC=1C(=C2C=NNC2=CC1)[N+](=O)[O-]
Catalog No.: TQP0943
Purity: 95%
MF: C7H4BrN3O2
MW: 242.032
Storage: 2-8 degree Celsius
SMILES: BrC=1C(=C2C=NNC2=CC1)[N+](=O)[O-]
CAS NO.: 2133005-85-1; 5-bromo-4-nitro-1H-indazole. PROPERTIES: 5-bromo-4-nitro-1H-indazole appears as a crystalline solid with characteristic halogen and nitro group reactivity. This aromatic heterocycle exhibits low solubility in water but dissolves well in polar aprotic solvents such as DMSO and DMF. Its molecular formula is C8H5BrN4O2, showing a molecular weight of approximately 261.05 g/mol. The compound demonstrates thermal stability up to 150 C but decomposes upon higher heating, releasing toxic fumes of bromine and nitrogen oxides. 5-bromo-4-nitro-1H-indazole must be stored between 2-8 degree Celsius in tightly sealed containers away from moisture and light. When handling this substance, protective equipment including gloves, eye protection, and lab coats is essential due to its potential irritant properties. Skin contact should be avoided, and in case of exposure, thorough washing with soap and water is recommended. The compound poses moderate fire hazard, and appropriate fire extinguishing equipment should be available. APPLICATIONS: 5-bromo-4-nitro-1H-indazole serves as a valuable building block in medicinal chemistry. Its bromine substituent allows for convenient cross-coupling reactions, while the nitro group can be reduced to amino functionality for further diversification. The indazole scaffold is prevalent in kinase inhibitors and anticancer agents, making this compound particularly useful in pharmaceutical discovery. Researchers utilize 5-bromo-4-nitro-1H-indazole derivatives in structure-activity relationship studies to optimize biological efficacy. Additionally, its photophysical properties enable applications in fluorescence-based assays after appropriate derivatization. The nitro-substituted indazole core has shown utility in bioisosteric replacements, enhancing metabolic stability of drug candidates. Proper storage at 2-8 C preserves its reactive functional groups for extended periods, ensuring consistent performance in synthetic protocols.
Reviews
Write Your Own Review