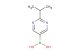
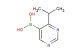
2-isopropyl-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyrimidine
$300.00
CAS No.: 1820679-54-6
Catalog No.: GS2544
Purity: 95%
MF: C13H21BN2O2
MW: 248.135
Storage: 2-8 degree Celsius
SMILES: C(C)(C)C1=NC=C(C=N1)B1OC(C(O1)(C)C)(C)C
Catalog No.: GS2544
Purity: 95%
MF: C13H21BN2O2
MW: 248.135
Storage: 2-8 degree Celsius
SMILES: C(C)(C)C1=NC=C(C=N1)B1OC(C(O1)(C)C)(C)C
CAS NO.: 1820679-54-6;2-isopropyl-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyrimidine. PROPERTIES: This pyrimidine boronate derivative appears as a pale yellow solid with a molecular weight of approximately 303.2 g/mol. The 2-isopropyl-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyrimidine combines a substituted pyrimidine ring with a boronate ester group. It exhibits limited aqueous solubility but good dissolution in polar aprotic solvents like THF and DMF. Stability characterization reveals vulnerability to hydrolysis and sensitivity to air moisture, necessitating storage at 2-8 degree Celsius in sealed glass containers under nitrogen. Handlers should use powder hoods with HEPA filtration and wear cut-resistant gloves during handling. Skin contact may cause mild irritation requiring thorough washing. Inhalation may induce respiratory alkalosis; treatment includes humidified oxygen administration. Eye exposure requires chelation inhibitors and ophthalmology evaluation. Waste should be hydrolyzed with dilute acid prior to disposal. APPLICATIONS: The 2-isopropyl-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyrimidine functions as a key intermediate in the synthesis of various pharmaceuticals and agrochemicals (excluding agricultural applications). Its boronate ester group provides a valuable handle for Suzuki-Miyaura cross-coupling reactions. Research teams utilize this compound as a starting material for creating pyrimidine-containing kinase inhibitors and nucleoside analogs. The isopropyl substituent offers enhanced solubility in lipid phases compared to methyl analogs. Additionally, the compound undergoes Buchwald-Hartwig amination for constructing anilinic systems. Its unique substitution pattern makes it valuable in the development of fluorescent probes and bioimaging agents.
Reviews
Write Your Own Review