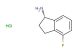
(1R)-1-(4-chlorophenyl)propan-1-amine hydrochloride
$350.00
CAS No.: 1448902-18-8
Catalog No.: WLZ0460
Purity: 95%
MF: C9H13Cl2N
MW: 206.116
Storage: 2-8 degree Celsius
SMILES: CC[C@H](C1=CC=C(Cl)C=C1)N.[H]Cl
Catalog No.: WLZ0460
Purity: 95%
MF: C9H13Cl2N
MW: 206.116
Storage: 2-8 degree Celsius
SMILES: CC[C@H](C1=CC=C(Cl)C=C1)N.[H]Cl
For R&D use only. Not for human or animal use.
CAS NO.: 1448902-18-8; (1R)-1-(4-chlorophenyl)propan-1-amine hydrochloride. PROPERTIES: (1R)-1-(4-chlorophenyl)propan-1-amine hydrochloride appears as white to off-white crystalline powder with a slight ammoniacal odor. Its molecular formula is C9H12ClN HCl, corresponding to a molecular weight of 209.11 g/mol. The compound has a melting point between 172-176 C and is highly soluble in water, forming clear solutions. Proper storage requires maintenance at 2-8 degree Celsius in tightly sealed containers to prevent deliquescence. When handling, use powder-free gloves and avoid inhalation of dust particles which may cause respiratory irritation. The substance is stable under dry conditions but hydrolyzes in aqueous environments to release the free amine and hydrochloric acid. It is classified as a mild irritant and should be managed in well-ventilated areas. The hydrochloride salt form enhances water solubility, facilitating formulation development for parenteral administration. The compound exhibits a moderate vapor pressure requiring careful handling to prevent volatilization losses. APPLICATIONS: (1R)-1-(4-chlorophenyl)propan-1-amine hydrochloride serves as a chiral amine building block in the synthesis of cardiovascular agents. The (1R)-configuration ensures proper spatial orientation of the substituents, which is critical for achieving optimal binding to adrenergic receptors. In medicinal chemistry, this compound is used to develop beta-blockers where the chlorophenyl group enhances membrane permeability, allowing for efficient crossing of the blood-brain barrier when necessary. The amine group provides a site for further functionalization, enabling the creation of prodrugs with improved pharmacokinetic profiles. Researchers in neuropharmacology utilize this compound to create selective adrenergic receptor antagonists, which are valuable tools for studying the role of these receptors in psychiatric disorders. The compound functions as a building block for synthesizing fluorescently labeled probes used to study receptor-ligand interactions in real-time. Additionally, derivatives of this compound are employed in the development of antihypertensive agents where the propylamine backbone facilitates interactions with angiotensin receptors. The hydrochloride salt form ensures good bioavailability when administered orally, making it suitable for chronic treatment regimens. The compound's structural features make it suitable for fragment-based drug discovery approaches, where its substructure can be elaborated into more complex molecules with optimized therapeutic indices. It also serves as a starting material for synthesizing enantiomerically pure amines through reductive amination reactions, providing access to a wide range of chiral secondary amines for pharmaceutical development.
Reviews
Write Your Own Review



![8-bromo-11-(1-methylpiperidin-4-ylidene)-6,11-dihydro-5H-benzo[5,6]cyclohepta[1,2-b]pyridine](https://www.chemshuttle.com/media/catalog/product/cache/31dbf0bffbfa69a5826a72cec9a446de/w/l/wlz0139_1.jpg)