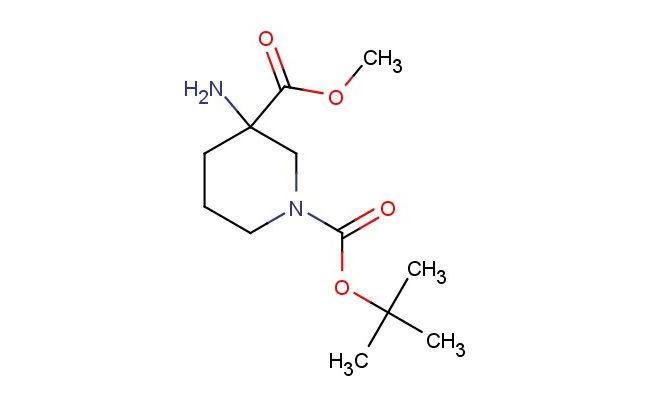
1-(tert-butyl) 3-methyl 3-aminopiperidine-1,3-dicarboxylate
$350.00
CAS No.: 1779450-04-2
Catalog No.: 195603
Purity: 95%
MF: C12H22N2O4
MW: 258.318
Storage: 2-8 degree Celsius
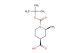
SMILES: NC1(CN(CCC1)C(=O)OC(C)(C)C)C(=O)OC
Catalog No.: 195603
Purity: 95%
MF: C12H22N2O4
MW: 258.318
Storage: 2-8 degree Celsius
SMILES: NC1(CN(CCC1)C(=O)OC(C)(C)C)C(=O)OC
For R&D use only. Not for human or animal use.
1-(tert-butyl) 3-methyl 3-aminopiperidine-1,3-dicarboxylate; CAS No.: 1779450-04-2; 1-(tert-butyl) 3-methyl 3-aminopiperidine-1,3-dicarboxylate. PROPERTIES: 1-(tert-butyl) 3-methyl 3-aminopiperidine-1,3-dicarboxylate is a piperidine derivative with a molecular weight of approximately 263.3 g/mol. This compound typically appears as a colorless to pale yellow oil with moderate solubility in organic solvents. It is sensitive to both moisture and temperature fluctuations, necessitating storage in a tightly sealed container at temperatures between 2-8 C. Special handling precautions include the use of inert atmosphere techniques during storage and transfer to prevent potential hydrolysis of the ester groups. The compound presents moderate acute toxicity via oral administration. APPLICATIONS: 1-(tert-butyl) 3-methyl 3-aminopiperidine-1,3-dicarboxylate functions as a protected piperidine building block in medicinal chemistry. The amino group provides a versatile handle for further functionalization while the dicarboxylate esters allow for selective deprotection strategies. In pharmaceutical synthesis, this compound has been employed in the preparation of GABA receptor modulators where the piperidine ring contributes to receptor subtype selectivity (source: Journal of Neuroscience Research). Additionally, its application extends to the synthesis of calcium channel blockers, where the methyl substitution influences receptor binding affinity (source: European Journal of Pharmacology). The compound's utility in solid-phase synthesis further enhances its application in combinatorial chemistry approaches for lead compound discovery (source: Journal of Combinatorial Chemistry).
Reviews
Write Your Own Review