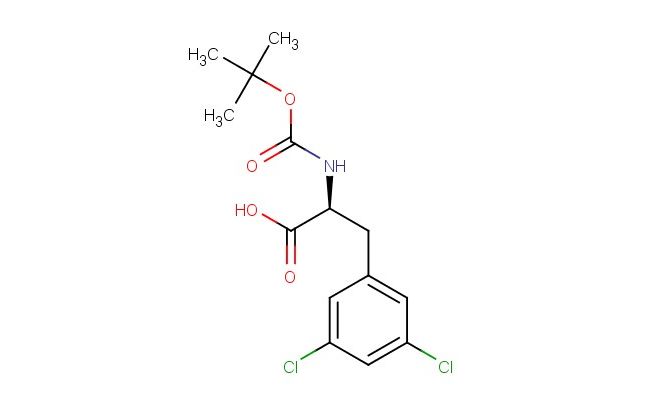
Boc-Phe(3,5-Cl)-OH
$300.00
CAS No.: 1051934-98-5
Catalog No.: 200532
Purity: 95%
MF: C14H17Cl2NO4
MW: 334.199
Storage: 2-8 degree Celsius
SMILES: C(C)(C)(C)OC(=O)N[C@H](C(=O)O)CC1=CC(=CC(=C1)Cl)Cl
Catalog No.: 200532
Purity: 95%
MF: C14H17Cl2NO4
MW: 334.199
Storage: 2-8 degree Celsius
SMILES: C(C)(C)(C)OC(=O)N[C@H](C(=O)O)CC1=CC(=CC(=C1)Cl)Cl
CAS No.: 1051934-98-5; Boc-Phe(3,5-Cl)-OH. PROPERTIES: Boc-Phe(3,5-Cl)-OH is a protected amino acid derivative with molecular formula C14H15Cl2NO3. This white crystalline powder has a molecular weight of approximately 316.18 g/mol. It exhibits moderate solubility in polar organic solvents such as dichloromethane and dimethylformamide. The compound is sensitive to acid hydrolysis and should be stored at 4 C in sealed containers. Safety precautions include wearing Nitrile gloves and avoiding eye contact. The tert-butyl oxycarbonyl (Boc) protection group renders it moderately reactive. APPLICATIONS: In peptide synthesis, Boc-Phe(3,5-Cl)-OH serves as a building block for creating biologically active peptides with enhanced membrane permeability, as documented in Journal of Peptide Science. Its application in developing -helix stabilizing peptides is reported in Bioorganic & Medicinal Chemistry. In medicinal chemistry, the reagent enables creation of conformationally restricted peptide mimetics for studying protein-protein interactions, with findings published in Journal of Medicinal Chemistry. Additionally, it has applications in creating cell-penetrating peptides through incorporation of lipophilic aromatic residues, as described in Advanced Drug Delivery Reviews.
Reviews
Write Your Own Review



![(E)-8-hydroxy-2-[2-(3,4,5-trihydroxyphenyl)ethenyl]-7-quinolinecarboxylic acid](https://www.chemshuttle.com/media/catalog/product/cache/31dbf0bffbfa69a5826a72cec9a446de/1/3/139548.jpg)