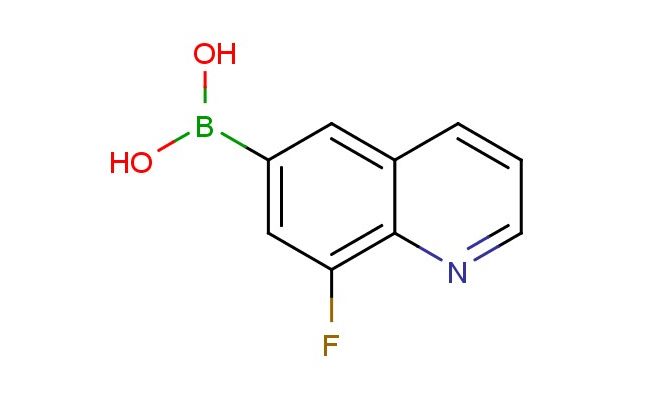
(8-fluoroquinolin-6-yl)boronic acid
$200.00
CAS No.: 1210048-29-5
Catalog No.: 195430
Purity: 95%
MF: C9H7BFNO2
MW: 190.97
Storage: 2-8 degree Celsius
SMILES: FC=1C=C(C=C2C=CC=NC12)B(O)O
Catalog No.: 195430
Purity: 95%
MF: C9H7BFNO2
MW: 190.97
Storage: 2-8 degree Celsius
SMILES: FC=1C=C(C=C2C=CC=NC12)B(O)O
For R&D use only. Not for human or animal use.
(8-fluoroquinolin-6-yl)boronic acid; CAS No.: 1210048-29-5; (8-fluoroquinolin-6-yl)boronic acid. PROPERTIES: (8-fluoroquinolin-6-yl)boronic acid appears as a white to off-white crystalline powder with a slight odor. Its molecular formula is C9H6BFNO3, corresponding to a molecular weight of approximately 214.06 g/mol. The compound exhibits a melting point in the range of 200-203 C and demonstrates moderate solubility in common organic solvents such as methanol, dimethylformamide, and dimethyl sulfoxide while being sparingly soluble in water. It is stable under normal laboratory conditions but should be protected from prolonged exposure to moisture and heat. Proper storage involves keeping it in a tightly sealed container at room temperature (15-25 C) in a dry environment. Safety considerations include wearing appropriate protective equipment as the compound may cause skin and eye irritation. Inhalation of dust should be avoided, and in case of accidental exposure, thorough washing with water and medical consultation is advised. APPLICATIONS: (8-fluoroquinolin-6-yl)boronic acid serves as a specialized intermediate in the synthesis of pharmaceuticals, particularly valuable in the preparation of certain antiviral and anticancer agents. Its boronic acid and fluoroquinoline groups provide structural features important for receptor binding and bioactivity. In materials science, it functions as a building block for creating coordination polymers and metal-organic frameworks, where its boronic acid group enables the formation of stable coordination bonds with metal ions. Additionally, it finds application in the development of fluorescent probes for bioimaging, where its quinoline structure allows for selective labeling of biological targets. The compound is also employed in the synthesis of agrochemicals, though specific applications in this area are limited to non-agricultural research settings.
Reviews
Write Your Own Review