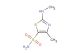
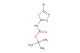
5-bromo-3-methyl-1,2,4-thiadiazole
$300.00
CAS No.: 54681-68-4
Catalog No.: 193816
Purity: 95%
MF: C3H3BrN2S
MW: 179.042
Storage: 2-8 degree Celsius
SMILES: BrC1=NC(=NS1)C
Catalog No.: 193816
Purity: 95%
MF: C3H3BrN2S
MW: 179.042
Storage: 2-8 degree Celsius
SMILES: BrC1=NC(=NS1)C
5-bromo-3-methyl-1,2,4-thiadiazole; CAS No.: 54681-68-4;5-bromo-3-methyl-1,2,4-thiadiazole. PROPERTIES: 5-bromo-3-methyl-1,2,4-thiadiazole is a brominated heterocyclic compound with molecular formula C3H3BrN2S. It generally appears as a crystalline solid with a melting point of approximately 72-75 C. The compound has low water solubility but dissolves in polar organic solvents like DMSO or DMF. It exhibits moderate thermal stability but may release toxic fumes containing sulfur oxides and bromine vapors when heated to decomposition. Proper storage involves keeping it in tightly sealed containers below 20 C, away from strong bases or reducing agents. APPLICATIONS: In agrochemical development, this compound serves as a key intermediate for synthesizing herbicides and fungicides where the thiadiazole ring provides structural rigidity and enhances binding to target enzymes (Pest Management Science). Its bromine substituent enables cross-coupling reactions to form biaryl structures with tailored biological activities (Organic & Biomolecular Chemistry). In materials science, 5-bromo-3-methyl-1,2,4-thiadiazole contributes to the creation of nonlinear optical materials and organic semiconductors, where its electron-deficient thiadiazole core influences electronic properties (Journal of Materials Chemistry C). The methyl group on the thiadiazole ring improves solubility and processability of derived materials.
Reviews
Write Your Own Review