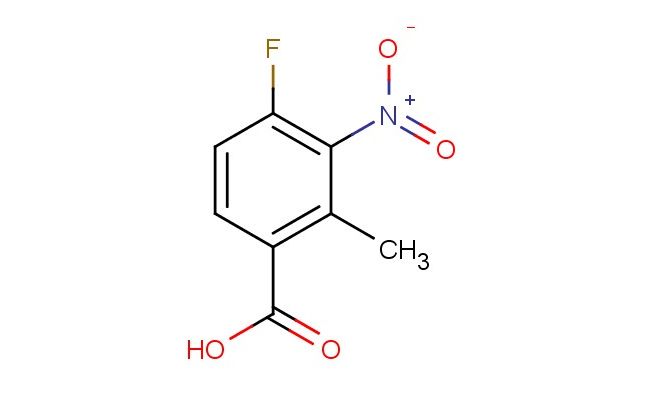
4-fluoro-2-methyl-3-nitrobenzoic acid
$250.00
CAS No.: 1079991-68-6
Catalog No.: WLZ1759
Purity: 95%
MF: C8H6FNO4
MW: 199.137
Storage: 2-8 degree Celsius
SMILES: FC1=C(C(=C(C(=O)O)C=C1)C)[N+](=O)[O-]
Catalog No.: WLZ1759
Purity: 95%
MF: C8H6FNO4
MW: 199.137
Storage: 2-8 degree Celsius
SMILES: FC1=C(C(=C(C(=O)O)C=C1)C)[N+](=O)[O-]
For R&D use only. Not for human or animal use.
CAS NO.: 1079991-68-6; 4-fluoro-2-methyl-3-nitrobenzoic acid. PROPERTIES: This fluorinated aromatic carboxylic acid features a fluorine atom, a methyl group, and a nitro group on a benzene ring connected to a carboxylic acid group, creating a molecule with potential applications in organic synthesis and pharmaceutical research. The 4-fluoro-2-methyl-3-nitrobenzoic acid typically appears as a white to off-white crystalline solid with moderate solubility in common organic solvents. Its molecular structure includes electron-withdrawing nitro and fluorine groups and an electron-donating methyl group that influence the electronic properties of the aromatic system. For optimal stability and to prevent degradation, this compound should be stored at 2-8 degree Celsius in a tightly sealed container under anhydrous conditions. When handling, appropriate safety measures including nitrile gloves and safety goggles are essential. This compound is sensitive to moisture and may hydrolyze in aqueous environments. In case of accidental spillage, clean the area with a damp cloth and dispose of materials according to local regulations. APPLICATIONS: The 4-fluoro-2-methyl-3-nitrobenzoic acid serves as a valuable intermediate in the synthesis of highly functionalized aromatic compounds and materials with specific electronic properties. The carboxylic acid group provides a handle for further functionalization through reactions such as esterification or amidation. In medicinal chemistry, this compound functions as a building block for developing pharmaceuticals targeting enzyme inhibitors and receptor modulators. The fluorine and methyl substituents contribute to target binding affinity and selectivity. Additionally, the molecule finds utility in materials science as a monomer for creating polymers with specific electronic and optical properties. Researchers utilizing this compound benefit from its defined substitution pattern, enabling the development of advanced materials with tailored electronic characteristics.
Reviews
Write Your Own Review