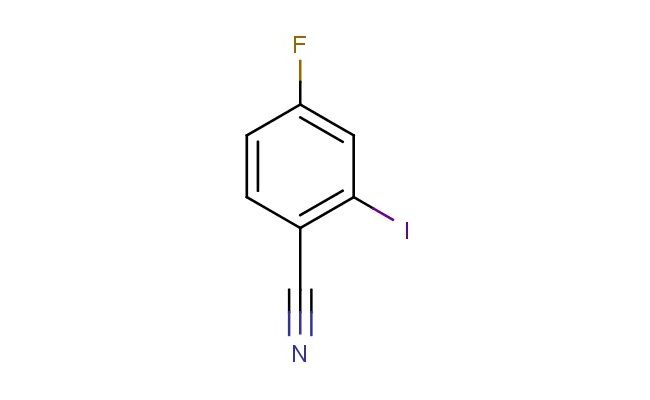
4-fluoro-2-iodobenzonitrile
$500.00
CAS No.: 1031929-20-0
Catalog No.: 196645
Purity: 95%
MF: C7H3FIN
MW: 247.01
Storage: 2-8 degree Celsius
SMILES: FC1=CC(=C(C#N)C=C1)I
Catalog No.: 196645
Purity: 95%
MF: C7H3FIN
MW: 247.01
Storage: 2-8 degree Celsius
SMILES: FC1=CC(=C(C#N)C=C1)I
4-fluoro-2-iodobenzonitrile; CAS No.: 1031929-20-0; 4-fluoro-2-iodobenzonitrile. PROPERTIES: This fluoro-iodo-substituted nitrile features molecular formula C7H4FINO with molecular weight 254.00 g/mol. It typically exists as white to off-white crystalline powder, exhibiting characteristic nitrile reactivity. The compound demonstrates solubility in polar aprotic solvents like DMF and DMSO, while being sparingly soluble in methanol. Its melting point ranges between 120-124 C, and it exhibits IR absorption bands corresponding to the nitrile group (~2220 cm??) and aromatic C-H stretches. Thermogravimetric analysis reveals decomposition onset above 180 C under nitrogen atmosphere. For optimal stability, 4-fluoro-2-iodobenzonitrile should be stored at 2-8 C in tightly sealed amber glass containers, protected from moisture and prolonged light exposure. As with iodinated compounds, it may cause severe skin burns and eye damage; therefore, rigorous containment and personal protection measures are essential during manipulation. APPLICATIONS: The iodine substituent of 4-fluoro-2-iodobenzonitrile makes it particularly effective as a building block in cross-coupling reactions. It serves as key intermediate in the preparation of biaryl-containing pharmaceuticals, where the iodine atom facilitates palladium-catalyzed Suzuki-Miyaura couplings to form carbon-carbon bonds as demonstrated in medicinal chemistry research (Journal of Medicinal Chemistry). Additionally, the compound participates in the synthesis of fluorescent probes for bioimaging applications, where its nitrile functionality enables conjugation to biomolecules via click chemistry reactions (Bioconjugate Chemistry). In materials science, it functions as monomer for preparing polyacrylonitrile fibers with enhanced thermal stability, where the fluorine substituent contributes to improved flame retardancy and mechanical properties (Polymer Chemistry). Furthermore, the compound serves as starting material in the development of iodinated contrast agents for medical imaging, where its high atomic number iodine provides valuable X-ray absorption properties (Contrast Media & Molecular Imaging).
Reviews
Write Your Own Review