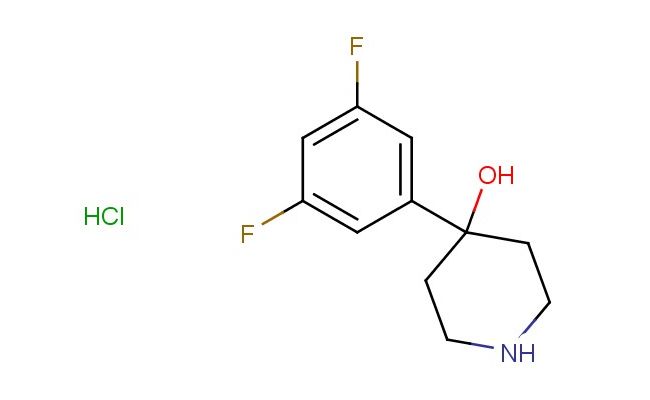
4-(3,5-Difluorophenyl)piperidin-4-ol hydrochloride
$350.00
CAS No.: 1146363-03-2
Catalog No.: 200377
Purity: 95%
MF: C11H14ClF2NO
MW: 249.688
Storage: 2-8 degree Celsius
SMILES: Cl.FC=1C=C(C=C(C1)F)C1(CCNCC1)O
Catalog No.: 200377
Purity: 95%
MF: C11H14ClF2NO
MW: 249.688
Storage: 2-8 degree Celsius
SMILES: Cl.FC=1C=C(C=C(C1)F)C1(CCNCC1)O
For R&D use only. Not for human or animal use.
CAS NO.: 1146363-03-2;4-(3,5-Difluorophenyl)piperidin-4-ol hydrochloride. PROPERTIES: This fluorinated amine salt presents as white hygroscopic powder with molecular weight approximately 273.7 g/mol (free base), combining piperidine nitrogen with vicinal hydroxyl and difluorophenyl groups. The 4-(3,5-Difluorophenyl)piperidin-4-ol hydrochloride exhibits good solubility in water and polar protic solvents but limited miscibility in non-polar media. Stability testing reveals tendency to form hydrates above 30% relative humidity, mandating storage at 2-8 degree Celsius in desiccated conditions with DRI-RITE indicators. Safety precautions include using deliquescence-resistant spatulas and maintaining relative humidity below 25% during handling. Inhalation may cause rhinitis and requires relocation to fresh air. Skin contact may lead to chemical burns in presence of moisture. Eye exposure necessitates 20 minutes of rinsing and immediate ophthalmology evaluation. Waste should be neutralized with sodium bicarbonate prior to disposal. APPLICATIONS: The 4-(3,5-Difluorophenyl)piperidin-4-ol hydrochloride functions as a key intermediate in the synthesis of dual orexin receptor antagonists and serotonin receptor modulators. Its piperidinol framework provides opportunities for reductive amination to construct tertiary amines. The compound serves as a building block for creating beta-adrenergic antagonists with enhanced receptor subtype selectivity. Additionally, it undergoes Mitsunobu reaction for inverting hydroxyl configuration in enantioselective synthesis. Research teams utilize it as a starting material for creating piperidine-based organocatalysts with defined stereochemistry. In materials science, its amine group enables formation of coordination polymers for gas storage applications.
Reviews
Write Your Own Review



![5,5-dimethyl-3-[4-(trifluoromethylsulfanyl)phenyl]imidazolidine-2,4-dione](https://www.chemshuttle.com/media/catalog/product/cache/31dbf0bffbfa69a5826a72cec9a446de/2/0/200373.jpg)