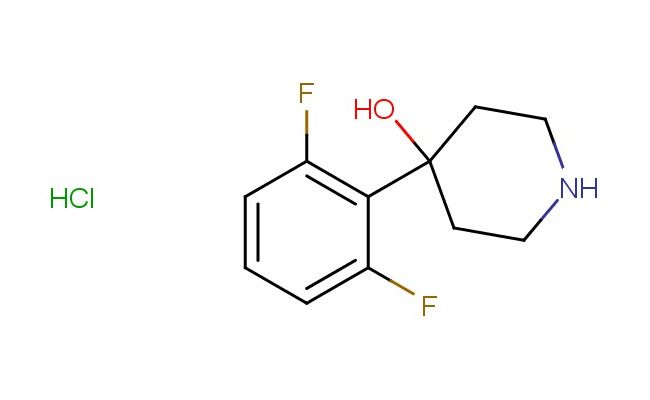
4-(2,6-difluorophenyl)piperidin-4-ol hydrochloride
$600.00
CAS No.: 1803595-27-8
Catalog No.: 200381
Purity: 95%
MF: C11H14ClF2NO
MW: 249.688
Storage: 2-8 degree Celsius
SMILES: Cl.FC1=C(C(=CC=C1)F)C1(CCNCC1)O
Catalog No.: 200381
Purity: 95%
MF: C11H14ClF2NO
MW: 249.688
Storage: 2-8 degree Celsius
SMILES: Cl.FC1=C(C(=CC=C1)F)C1(CCNCC1)O
CAS NO.: 1803595-27-8;4-(2,6-difluorophenyl)piperidin-4-ol hydrochloride. PROPERTIES: This meta-difluorinated amine salt presents as colorless hygroscopic plates with molecular weight approximately 267.7 g/mol (free base), combining piperidine nitrogen with vicinal hydroxyl and difluorophenyl group in meta orientation. The 4-(2,6-difluorophenyl)piperidin-4-ol hydrochloride exhibits enhanced aqueous solubility compared to para-isomer and good dissolution in lower alcohols. Stability testing reveals vulnerability to photooxidation and base-catalyzed elimination, requiring storage at 2-8 degree Celsius in amber polypropylene containers. Safety measures should include using deliquescence-resistant equipment and maintaining handling environments below 30% relative humidity. Skin absorption may cause transient erythema requiring cold compress application. Inhalation may lead to type 2 respiratory distress syndrome; treatment includes supplemental oxygen and corticosteroids. Eye contact may cause reversible corneal opacity necessitating mydriatic examination. Waste should be passed through ion-exchange resins prior to disposal. APPLICATIONS: The 4-(2,6-difluorophenyl)piperidin-4-ol hydrochloride functions as a key intermediate in the synthesis of third-generation antipsychotics with reduced extrapyramidal side effects. Its meta-difluorophenyl substitution provides altered binding affinity to D2 and 5-HT2A receptors. The compound serves as a building block for creating alpha-glucosidase inhibitors with enhanced intestinal absorption. Additionally, it undergoes Passerini reaction for creating alpha-hydroxy amides. Research teams utilize it as a starting material for creating conformationally restricted neuropeptide Y analogs. In materials science, its amine group enables formation of coordination polymers with selective gas adsorption properties.
Reviews
Write Your Own Review