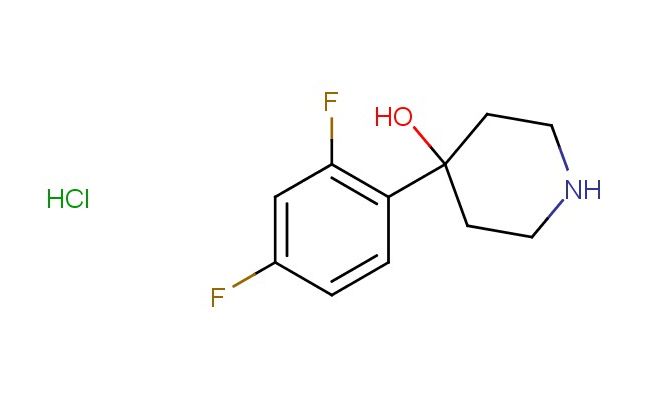
4-(2,4-difluorophenyl)piperidin-4-ol hydrochloride
$400.00
CAS No.: 2832911-54-1
Catalog No.: 200379
Purity: 95%
MF: C11H14ClF2NO
MW: 249.688
Storage: 2-8 degree Celsius
SMILES: Cl.FC1=C(C=CC(=C1)F)C1(CCNCC1)O
Catalog No.: 200379
Purity: 95%
MF: C11H14ClF2NO
MW: 249.688
Storage: 2-8 degree Celsius
SMILES: Cl.FC1=C(C=CC(=C1)F)C1(CCNCC1)O
For R&D use only. Not for human or animal use.
CAS NO.: 2832911-54-1;4-(2,4-difluorophenyl)piperidin-4-ol hydrochloride. PROPERTIES: This difluorinated amine salt appears as off-white hygroscopic crystals with molecular weight approximately 267.7 g/mol (free base), combining piperidine nitrogen with vicinal hydroxyl and difluorophenyl substituent. The 4-(2,4-difluorophenyl)piperidin-4-ol hydrochloride demonstrates moderate aqueous solubility with pH-dependent speciation and good dissolution in methanol. Stability characterization reveals susceptibility to base-catalyzed Hofmann elimination and hydrolysis above pH 9, necessitating storage at 2-8 degree Celsius in sealed polyethylene containers with dessicants. Handlers should employ deliquescence-resistant tools and maintain environmental humidity below 35%. Skin contact in presence of moisture may cause mild irritation requiring thorough washing. Inhalation may induce bronchial spasms; treatment includes bronchodilator administration. Eye exposure requires extended rinsing and possible corticosteroid application. Waste should be processed through activated alumina before disposal. APPLICATIONS: The 4-(2,4-difluorophenyl)piperidin-4-ol hydrochloride serves as a versatile intermediate in the preparation of kinase inhibitors targeting FLT3 and JAK family enzymes. Its piperidinol core enables reductive amination to form tertiary amine pharmacophores. The compound functions as a key building block in the synthesis of dual NHEP1/2 inhibitors. Additionally, it undergoes Staudinger reaction for creating phosphorus-containing heterocycles. Research teams employ it as a chiral auxiliary in asymmetric hydrogenation processes. In materials science, its amine group facilitates formation of metal-organic frameworks with tunable porosity for catalytic applications.
Reviews
Write Your Own Review