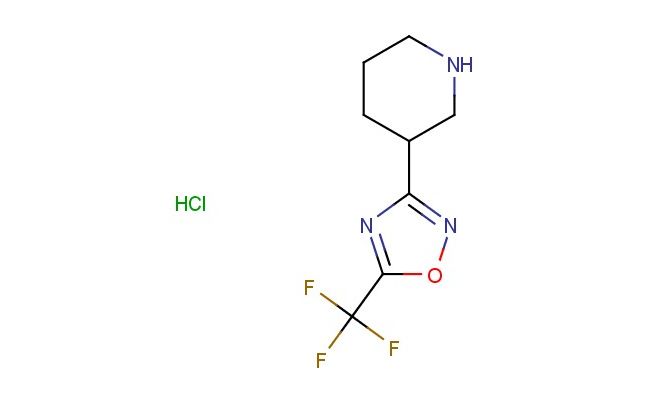
3-(piperidin-3-yl)-5-(trifluoromethyl)-1,2,4-oxadiazole hydrochloride
$400.00
CAS No.: 1315610-08-2
Catalog No.: LT0024
Purity: 95%
MF: C8H11ClF3N3O
MW: 257.643
Storage: 2-8 degree Celsius
SMILES: Cl.N1CC(CCC1)C1=NOC(=N1)C(F)(F)F
Catalog No.: LT0024
Purity: 95%
MF: C8H11ClF3N3O
MW: 257.643
Storage: 2-8 degree Celsius
SMILES: Cl.N1CC(CCC1)C1=NOC(=N1)C(F)(F)F
For R&D use only. Not for human or animal use.
CAS NO.: 1315610-08-2;3-(piperidin-3-yl)-5-(trifluoromethyl)-1,2,4-oxadiazole hydrochloride. PROPERTIES: This piperidine oxadiazole derivative salt appears as a white hygroscopic powder with a molecular weight of approximately 256.7 g/mol (free base). The 3-(piperidin-3-yl)-5-(trifluoromethyl)-1,2,4-oxadiazole hydrochloride combines a piperidine ring with an oxadiazole framework and trifluoromethyl group. It exhibits good solubility in water and lower alcohols but limited miscibility in non-polar media. Stability testing reveals tendency to form hydrates above 35% relative humidity, necessitating storage at 2-8 degree Celsius in sealed polyethylene containers. Handlers should employ deliquescence-resistant tools and maintain environmental humidity below 30%. Skin contact may cause chemical burns in presence of moisture. Inhalation may induce bronchial hyperreactivity; treatment includes anticholinergic inhalers. Eye exposure requires extended rinsing and possible corticosteroid application. Waste should be neutralized with sodium bicarbonate prior to disposal. APPLICATIONS: The 3-(piperidin-3-yl)-5-(trifluoromethyl)-1,2,4-oxadiazole hydrochloride serves as a key intermediate in the synthesis of various pharmaceuticals. Its oxadiazole framework provides opportunities for click chemistry reactions. Research teams utilize this compound as a starting material for creating kinase inhibitors and muscarinic receptor modulators. The piperidine group undergoes oxidation to form more complex ring systems. Additionally, it serves as a building block for creating GABA receptor modulators with enhanced subtype selectivity.
Reviews
Write Your Own Review