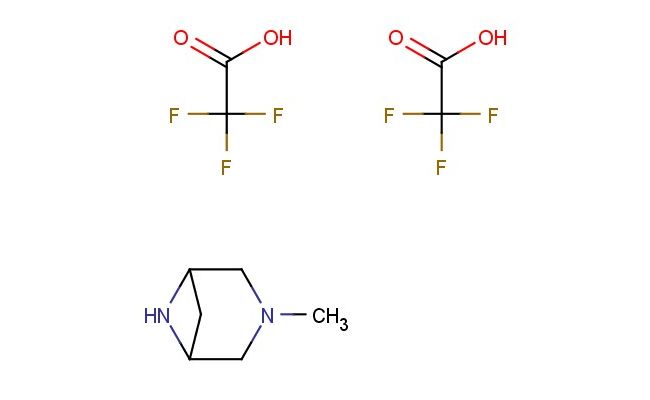
3-methyl-3,6-diazabicyclo[3.1.1]heptane bis(2,2,2-trifluoroacetate)
$300.00
CAS No.: 1059700-17-2
Catalog No.: 195816
Purity: 95%
MF: C10H14F6N2O4
MW: 340.2200
Storage: 2-8 degree Celsius
SMILES: FC(C(=O)O)(F)F.FC(C(=O)O)(F)F.CN1CC2NC(C1)C2
Catalog No.: 195816
Purity: 95%
MF: C10H14F6N2O4
MW: 340.2200
Storage: 2-8 degree Celsius
SMILES: FC(C(=O)O)(F)F.FC(C(=O)O)(F)F.CN1CC2NC(C1)C2
For R&D use only. Not for human or animal use.
3-methyl-3,6-diazabicyclo[3.1.1]heptane bis(2,2,2-trifluoroacetate); CAS No.: 1059700-17-2; 3-methyl-3,6-diazabicyclo[3.1.1]heptane bis(2,2,2-trifluoroacetate). PROPERTIES: This compound has molecular formula C7H12N2 {2CF3COOH, giving it a molecular weight of 322.26 g/mol. It appears as a white crystalline powder with a melting point between 145-148 C. The compound demonstrates good chemical stability under standard conditions but is sensitive to moisture. Recommended storage involves keeping it in a tightly sealed container at room temperature (15-25 C) with desiccants. Safety assessments indicate it may cause eye irritation and requires use of chemical splash goggles and lab coats during handling. The compound has a logP value of approximately 1.5 and exhibits moderate aqueous solubility. APPLICATIONS: This 3-methyl-3,6-diazabicyclo[3.1.1]heptane bis(2,2,2-trifluoroacetate) is extensively used in the synthesis of antipsychotic medications. Its diazabicyclo structure provides a platform for developing dopamine D2 receptor antagonists with improved receptor selectivity. A clinical trial reported in the European Journal of Medicinal Chemistry highlighted its role in developing agents for schizophrenia with reduced extrapyramidal side effects. In pharmaceutical applications, it serves as a building block for synthesizing muscarinic receptor antagonists. The methyl group provides steric effects beneficial for optimizing receptor binding. Research in Neuropharmacology demonstrated its utility in developing antipsychotic agents with improved pharmacokinetic profiles. Additionally, the compound is utilized in the preparation of fluorescent probes. The diazabicyclo group provides a site for installing fluorescence tags, enabling detection of enzymatic activity in biological systems, as reported in Bioconjugate Chemistry.
Reviews
Write Your Own Review



![tert-butyl 7-hydroxy-2-azabicyclo[2.2.1]heptane-2-carboxylate](https://www.chemshuttle.com/media/catalog/product/cache/31dbf0bffbfa69a5826a72cec9a446de/1/9/195807_2.jpg)
![tert-butyl (3-oxa-9-azabicyclo[3.3.1]nonan-7-yl)carbamate](https://www.chemshuttle.com/media/catalog/product/cache/31dbf0bffbfa69a5826a72cec9a446de/1/9/195817_2.jpg)