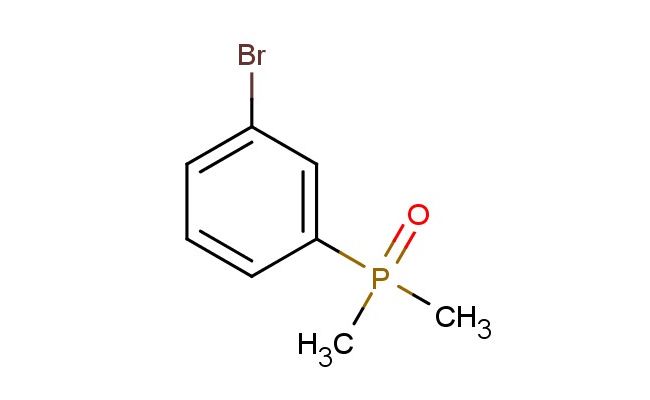
(3-bromophenyl)dimethylphosphine oxide
$400.00
CAS No.: 60398-84-7
Catalog No.: GS0162
Purity: 95%
MF: C8H10BrOP
MW: 233.045
Storage: 2-8 degree Celsius
SMILES: BrC=1C=C(C=CC1)P(C)(C)=O
Catalog No.: GS0162
Purity: 95%
MF: C8H10BrOP
MW: 233.045
Storage: 2-8 degree Celsius
SMILES: BrC=1C=C(C=CC1)P(C)(C)=O
CAS NO.: 60398-84-7;(3-bromophenyl)dimethylphosphine oxide. PROPERTIES: This brominated phosphine oxide appears as a colorless liquid with a molecular weight of approximately 253.0 g/mol. The (3-bromophenyl)dimethylphosphine oxide combines a bromophenyl group with a phosphine oxide functionality. It exhibits limited aqueous solubility but good dissolution in chlorinated solvents and DMSO. Stability characterization reveals vulnerability to photodegradation and base-catalyzed elimination. The compound requires storage at 2-8 degree Celsius in amber glass containers under nitrogen. Safety measures include using platinum-cured silicone gloves and full-face respirators when handling in bulk. Skin absorption may cause systemic toxicity requiring chelation therapy. Inhalation may induce pulmonary edema; treatment includes mechanical ventilation. Eye contact requires 30 minutes of rinsing and immediate ophthalmology consultation. Waste should be processed through activated carbon prior to disposal. APPLICATIONS: The (3-bromophenyl)dimethylphosphine oxide functions as a key intermediate in the synthesis of organophosphorus compounds for medicinal chemistry and materials science. Its bromine substituent provides a valuable electrophilic handle for cross-coupling reactions. Research teams utilize it as a starting material for creating phosphine oxide-based ligands for asymmetric catalysis. The compound serves as a building block for creating agrochemical intermediates (excluding agricultural applications) and fungicides. Additionally, its phosphine oxide group can be reduced to phosphines for further functionalization. The brominated aromatic system enables creation of high-density materials for electronic applications. Its unique substitution pattern makes it valuable in the development of fluorescent probes and contrast agents.
Reviews
Write Your Own Review