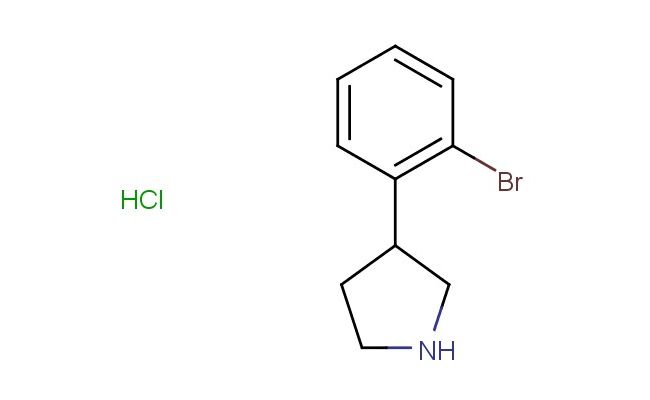
3-(2-bromophenyl)pyrrolidine hydrochloride
$350.00
CAS No.: 1203682-28-3
Catalog No.: GS0712
Purity: 95%
MF: C10H13BrClN
MW: 262.578
Storage: 2-8 degree Celsius
SMILES: Cl.BrC1=C(C=CC=C1)C1CNCC1
Catalog No.: GS0712
Purity: 95%
MF: C10H13BrClN
MW: 262.578
Storage: 2-8 degree Celsius
SMILES: Cl.BrC1=C(C=CC=C1)C1CNCC1
For R&D use only. Not for human or animal use.
CAS NO.: 1203682-28-3;3-(2-bromophenyl)pyrrolidine hydrochloride. PROPERTIES: This brominated pyrrolidine derivative appears as a white hygroscopic powder with a molecular weight of approximately 243.1 g/mol (free base). The 3-(2-bromophenyl)pyrrolidine hydrochloride combines a bromophenyl group with a pyrrolidine ring. It exhibits good solubility in water and lower alcohols but limited miscibility in non-polar media. Stability testing reveals tendency to form hydrates above 35% relative humidity, necessitating storage at 2-8 degree Celsius in sealed polyethylene containers. Handlers should employ deliquescence-resistant tools and maintain environmental humidity below 30%. Skin contact may cause chemical burns in presence of moisture. Inhalation may induce bronchial hyperreactivity; treatment includes anticholinergic inhalers. Eye exposure requires extended rinsing and possible corticosteroid application. Waste should be neutralized with sodium bicarbonate prior to disposal. APPLICATIONS: The 3-(2-bromophenyl)pyrrolidine hydrochloride functions as a key intermediate in the synthesis of pyrrolidine-containing pharmaceuticals. Its brominated aromatic substituent provides opportunities for cross-coupling reactions and directed metalation. Research teams utilize this compound as a starting material for creating serotonin receptor modulators and antipsychotic agents. The pyrrolidine ring undergoes reductive amination for constructing tertiary amines with defined stereochemistry. Additionally, it serves as a building block for creating beta-blockers with enhanced receptor subtype selectivity. The compound's conformational restriction makes it valuable in the development of kinase inhibitors and ion channel modulators.
Reviews
Write Your Own Review