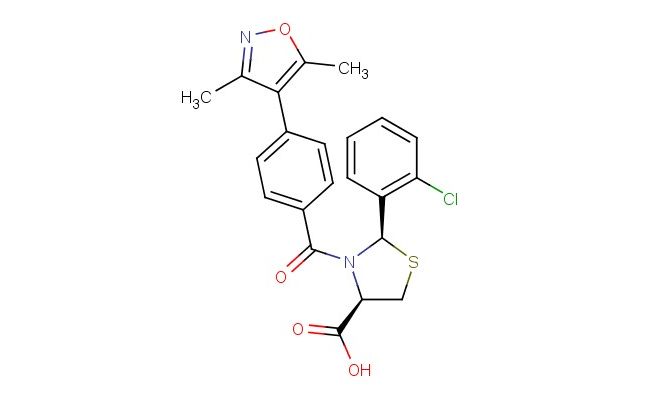
(2R,4R)-2-(2-chlorophenyl)-3-(4-(3,5-dimethylisoxazol-4-yl)benzoyl)thiazolidine-4-carboxylic acid
$300.00
CAS No.: 2247372-59-2
Catalog No.: TQR0323
Purity: 95%
MF: C22H19ClN2O4S
MW: 442.924
Storage: 2-8 degree Celsius
SMILES: ClC1=C(C=CC=C1)[C@H]1SC[C@H](N1C(C1=CC=C(C=C1)C=1C(=NOC1C)C)=O)C(=O)O
Catalog No.: TQR0323
Purity: 95%
MF: C22H19ClN2O4S
MW: 442.924
Storage: 2-8 degree Celsius
SMILES: ClC1=C(C=CC=C1)[C@H]1SC[C@H](N1C(C1=CC=C(C=C1)C=1C(=NOC1C)C)=O)C(=O)O
CAS NO.: 2247372-59-2; (2R,4R)-2-(2-chlorophenyl)-3-(4-(3,5-dimethylisoxazol-4-yl)benzoyl)thiazolidine-4-carboxylic acid. PROPERTIES: (2R,4R)-2-(2-chlorophenyl)-3-(4-(3,5-dimethylisoxazol-4-yl)benzoyl)thiazolidine-4-carboxylic acid appears as white to off-white crystalline powder with a slight chloride odor. Its molecular formula is C23H18ClN3O4S, corresponding to a molecular weight of 481.92 g/mol. The compound demonstrates limited solubility in water but dissolves in DMSO and DMF with moderate ease. Proper storage requires temperatures between 2-8 degree Celsius in amber glass containers to protect against light sensitivity and moisture. When handling, use chemical-resistant gloves and safety goggles to prevent skin and eye contact, which may cause mild irritation. The substance is stable under dry, cool conditions but hydrolyzes in aqueous environments, releasing the corresponding amine and carboxylic acid. APPLICATIONS: (2R,4R)-2-(2-chlorophenyl)-3-(4-(3,5-dimethylisoxazol-4-yl)benzoyl)thiazolidine-4-carboxylic acid serves as a key intermediate in the synthesis of dual enzyme inhibitors targeting kinases and proteases. The thiazolidine core provides a flexible scaffold that can accommodate multiple hydrogen bonding interactions within enzyme active sites. In medicinal chemistry, this compound is used to develop anticancer agents where the chlorophenyl group enhances membrane permeability, while the isoxazole substituent modulates target selectivity. The carboxylic acid group allows for further functionalization, enabling the creation of prodrugs with improved pharmacokinetic properties. Researchers in structural biology utilize this compound to crystallize enzyme-inhibitor complexes, facilitating the determination of binding modes through X-ray diffraction studies. Additionally, the compound functions as a building block for synthesizing fluorescently labeled probes used to study enzyme kinetics in real-time. The stereospecific (2R,4R) configuration ensures proper orientation within the binding pocket, maximizing inhibitory efficacy against specific isoforms of target enzymes.
Reviews
Write Your Own Review



![4-(((1r,4r)-4-hydroxycyclohexyl)amino)-4'-isopropyl-[1,1'-biphenyl]-3-carboxamide](https://www.chemshuttle.com/media/catalog/product/cache/31dbf0bffbfa69a5826a72cec9a446de/t/q/tqr0320_1.jpg)