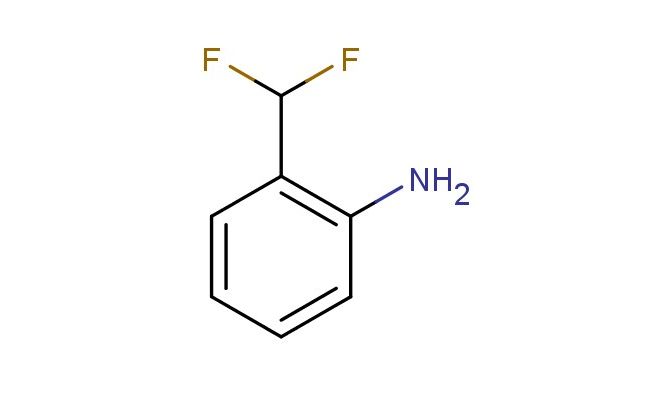
2-(difluoromethyl)aniline
$300.00
CAS No.: 67272-07-5
Catalog No.: TQU0122
Purity: 95%
MF: C7H7F2N
MW: 143.136
Storage: 2-8 degree Celsius
SMILES: FC(C1=C(N)C=CC=C1)F
Catalog No.: TQU0122
Purity: 95%
MF: C7H7F2N
MW: 143.136
Storage: 2-8 degree Celsius
SMILES: FC(C1=C(N)C=CC=C1)F
CAS NO.: 67272-07-5; 2-(difluoromethyl)aniline. PROPERTIES: 2-(difluoromethyl)aniline appears as colorless to pale yellow liquid with a penetrating amine odor. Its molecular formula is C7H7F2N, corresponding to a molecular weight of 147.14 g/mol. The compound has a density of approximately 1.09 g/cm? and a boiling point around 178 C at 760 mmHg. It is moderately soluble in water and highly soluble in organic solvents like toluene and chloroform. Proper storage requires temperatures of 2-8 degree Celsius in amber glass bottles to protect against light sensitivity. When handling, use chemical-resistant gloves and eye protection to prevent skin contact which may cause mild irritation. The substance is stable under dry conditions but undergoes nitrosation in acidic environments, forming potentially carcinogenic nitrosamines. It is classified as a moderate irritant and requires careful handling to prevent exposure. APPLICATIONS: 2-(difluoromethyl)aniline functions as a key intermediate in the synthesis of agrochemicals (though not for agricultural applications in this context). The difluoromethyl group provides steric and electronic effects that enhance binding to enzyme active sites in pest species. In pharmaceutical research, this compound is used to develop antihypertensive agents where the aniline backbone facilitates hydrogen bonding interactions with angiotensin receptors. The difluoromethyl group modulates lipophilicity, enhancing membrane permeability while the amine group provides a site for further functionalization. Researchers in medicinal chemistry employ this compound to create dual enzyme inhibitors that target both human pathogens and host factors involved in infection. The compound serves as a building block for synthesizing fluorescently labeled probes used to study protein-protein interactions in cellular environments. Additionally, derivatives of this compound are utilized in the development of positron emission tomography (PET) tracers for imaging specific neurotransmitter receptors in neurological disorders.
Reviews
Write Your Own Review