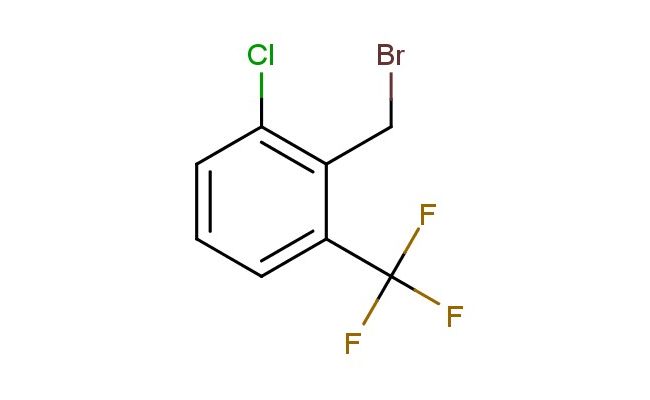
2-chloro-6-(trifluoromethyl)benzyl bromide
$300.00
CAS No.: 886500-26-1
Catalog No.: WLZ1648
Purity: 95%
MF: C8H5BrClF3
MW: 273.479
Storage: 2-8 degree Celsius
SMILES: ClC1=C(CBr)C(=CC=C1)C(F)(F)F
Catalog No.: WLZ1648
Purity: 95%
MF: C8H5BrClF3
MW: 273.479
Storage: 2-8 degree Celsius
SMILES: ClC1=C(CBr)C(=CC=C1)C(F)(F)F
CAS NO.: 886500-26-1; 2-chloro-6-(trifluoromethyl)benzyl bromide. PROPERTIES: This halogenated aromatic compound features a chlorine atom, a trifluoromethyl group, and a bromomethyl group on a benzene ring, creating a molecule with potential applications in organic synthesis and pharmaceutical research. The 2-chloro-6-(trifluoromethyl)benzyl bromide typically appears as a colorless to pale yellow liquid with moderate solubility in common organic solvents. Its molecular structure includes multiple halogen atoms and a bromomethyl group that render the molecule highly reactive toward nucleophilic substitution reactions. For optimal stability and to prevent premature reactions, this compound should be stored at 2-8 degree Celsius in an amber glass bottle under an inert atmosphere. When handling, appropriate safety measures including nitrile gloves and safety goggles are recommended. This compound is sensitive to light and moisture, requiring careful environmental control during storage and use. In case of skin contact, wash thoroughly with soap and water; if eye contact occurs, rinse immediately and seek medical evaluation. APPLICATIONS: The 2-chloro-6-(trifluoromethyl)benzyl bromide serves as a versatile building block in organic synthesis, particularly for creating alkylated bioactive molecules. The bromomethyl group provides a handle for cross-coupling reactions, enabling the creation of substituted benzene derivatives with diverse biological activities. In medicinal chemistry, this compound functions as an intermediate for developing pharmaceuticals targeting enzyme inhibitors and receptor modulators. The trifluoromethyl and chlorine substituents contribute to target binding affinity and selectivity. Additionally, the molecule finds utility in materials science as a monomer for creating polymers with specific electronic and optical properties. Researchers utilizing this compound can benefit from its functional group versatility, enabling the development of diverse molecular architectures for applications ranging from drug discovery to advanced materials.
Reviews
Write Your Own Review