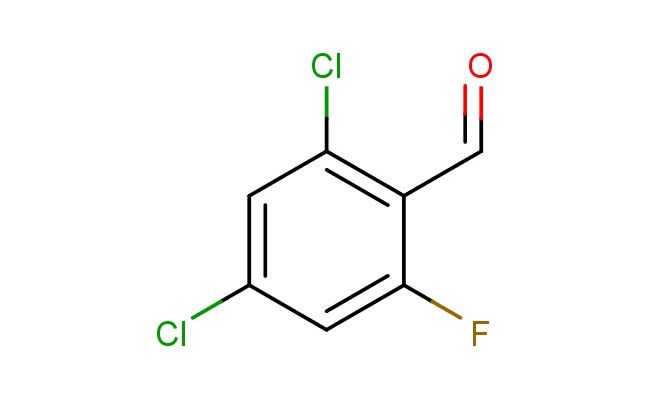
2,4-dichloro-6-fluorobenzaldehyde
$250.00
CAS No.: 681435-09-6
Catalog No.: 196642
Purity: 95%
MF: C7H3Cl2FO
MW: 193.004
Storage: 2-8 degree Celsius
SMILES: ClC1=C(C=O)C(=CC(=C1)Cl)F
Catalog No.: 196642
Purity: 95%
MF: C7H3Cl2FO
MW: 193.004
Storage: 2-8 degree Celsius
SMILES: ClC1=C(C=O)C(=CC(=C1)Cl)F
2,4-dichloro-6-fluorobenzaldehyde; CAS No.: 681435-09-6; 2,4-dichloro-6-fluorobenzaldehyde. PROPERTIES: This dichloro-fluoro-substituted aldehyde possesses molecular formula C7H4Cl2FO with molecular weight 193.00 g/mol. It typically presents as colorless to pale yellow liquid, exhibiting characteristic aldehyde reactivity. The compound demonstrates solubility in polar aprotic solvents like ethyl acetate and dichloromethane, while being sparingly soluble in water. Its boiling point ranges between 140-145 C at 760 mmHg, and it exhibits IR absorption bands corresponding to the aldehyde group (~2800-2600 cm??) and aromatic C-H stretches. Thermogravimetric analysis indicates decomposition onset above 150 C under nitrogen atmosphere. For optimal stability, 2,4-dichloro-6-fluorobenzaldehyde should be stored at 2-8 C in tightly sealed amber glass containers, protected from moisture and prolonged light exposure. As with aldehyde compounds, it may cause severe skin burns and eye damage; therefore, rigorous containment and personal protection measures are essential during manipulation. APPLICATIONS: As a multifunctional aromatic aldehyde, 2,4-dichloro-6-fluorobenzaldehyde is predominantly utilized in the synthesis of agrochemicals. It serves as key intermediate in constructing phenylpyrazoline herbicides, where the aldehyde group undergoes cyclization reactions to form the characteristic five-membered heterocycle as demonstrated in pesticide chemistry research (Pest Management Science). Additionally, the compound participates in the preparation of fluorescent probes for bioimaging applications, where its aldehyde functionality enables conjugation to primary amines in biomolecules via Schiff base formation (Bioconjugate Chemistry). In pharmaceutical development, it functions as building block in the synthesis of histamine receptor antagonists, where the chloro and fluoro substituents provide valuable binding affinity and selectivity (European Journal of Medicinal Chemistry). Furthermore, the compound serves as starting material in the development of aldehyde-based crosslinking agents for protein immobilization, where its reactive aldehyde group forms covalent bonds with amino acid side chains (ACS Applied Materials & Interfaces).
Reviews
Write Your Own Review