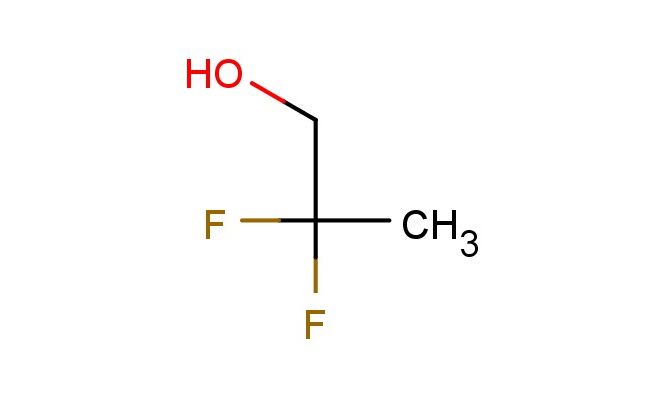
2,2-difluoropropan-1-ol
$200.00
CAS No.: 33420-52-9
Catalog No.: TQU0001
Purity: 95%
MF: C3H6F2O
MW: 96.076
Storage: 2-8 degree Celsius
SMILES: FC(CO)(C)F
Catalog No.: TQU0001
Purity: 95%
MF: C3H6F2O
MW: 96.076
Storage: 2-8 degree Celsius
SMILES: FC(CO)(C)F
For R&D use only. Not for human or animal use.
CAS NO.: 33420-52-9; 2,2-difluoropropan-1-ol. PROPERTIES: 2,2-difluoropropan-1-ol appears as a colorless liquid with a sharp, pungent odor. Its molecular formula is C3H6F2O, corresponding to a molecular weight of 94.07 g/mol. The compound has a density of approximately 1.15 g/cm? and a boiling point around 65 C at 760 mmHg. It is miscible with most organic solvents and has moderate water solubility. Proper storage requires temperatures of 2-8 degree Celsius in glass containers with tight-sealing caps to prevent evaporation. When handling, use chemical-resistant gloves and eye protection to prevent skin and eye contact, which may cause mild irritation. The substance is flammable and requires storage away from heat and ignition sources. It is stable under normal laboratory conditions but decomposes upon prolonged exposure to strong acids or bases. APPLICATIONS: 2,2-difluoropropan-1-ol serves as a versatile building block in organic synthesis. The difluoro substitution provides unique reactivity, enabling the formation of stable carbon-oxygen bonds in subsequent reactions. In pharmaceutical research, this compound is used to develop antimicrobial agents where the fluorinated alcohol group enhances penetration through bacterial cell membranes. The compound functions as a chiral auxiliary in asymmetric synthesis, directing the formation of specific stereoisomers during nucleophilic addition reactions. In materials science, derivatives of this compound are employed in the synthesis of fluorinated polymers with improved thermal stability and chemical resistance. Researchers in agrochemical development utilize this compound (though not for agricultural applications) to synthesize plant growth regulators with novel modes of action, exploiting the compound's ability to modulate hormonal pathways in botanical systems. Additionally, the compound is used as a solvent in specific chemical reactions where its polarity and fluorinated character provide unique solvation properties that enhance reaction rates and selectivities.
Reviews
Write Your Own Review