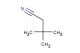
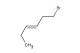
1-Isocyanato-2-methoxyethane
$400.00
CAS No.: 42170-95-6
Catalog No.: 200363
Purity: 95%
MF: C4H7NO2
MW: 101.105
Storage: 2-8 degree Celsius
SMILES: N(=C=O)CCOC
Catalog No.: 200363
Purity: 95%
MF: C4H7NO2
MW: 101.105
Storage: 2-8 degree Celsius
SMILES: N(=C=O)CCOC
For R&D use only. Not for human or animal use.
CAS NO.: 42170-95-6;1-Isocyanato-2-methoxyethane. PROPERTIES: This reactive intermediate presents as a colorless liquid with distinctive aromatic odor, exhibiting good solubility in ether and THF but forming hydrogen bonds with protic solvents. The 1-Isocyanato-2-methoxyethane has a molecular weight of approximately 111.1 g/mol, combining methoxyethyl backbone with terminal isocyanate group. Stability analysis demonstrates sensitivity to moisture and atmospheric CO2, requiring storage below 8 degree Celsius in moisture-proof containers with molecular sieves. Essential safety measures include using PTFE-lined septa and maintaining negative pressure in transfer lines. Skin exposure may lead to protein crosslinking and requires immediate washing with acetone followed by thorough rinsing. Inhalation poses risk of pulmonary edema, necessitating immediate fresh air and medical monitoring. In case of fire, carbon dioxide extinguishers should not be used due to potential phosgene formation. Waste neutralization with alcohols should be conducted in controlled manner. APPLICATIONS: The 1-Isocyanato-2-methoxyethane serves as a chain extender in polyurethane foam production through reaction with polyols. Its methoxyethyl segment provides enhanced flexibility in resulting polymer networks. In chemical synthesis, it acts as a methoxyethyl isocyanate equivalent in urethane protection of hydroxyl groups. The compound enables selective modification of primary amines in peptide synthesis through steric hindrance effects. Additionally, it functions as a building block for carbamate insecticides (excluding agricultural applications) and fungicides. In specialty coatings, the bifunctional nature allows creation of crosslinked films with improved adhesion properties. Research teams utilize it for modifying nanoparticles surfaces through isocyanate reactions with surface hydroxyl groups.
Reviews
Write Your Own Review