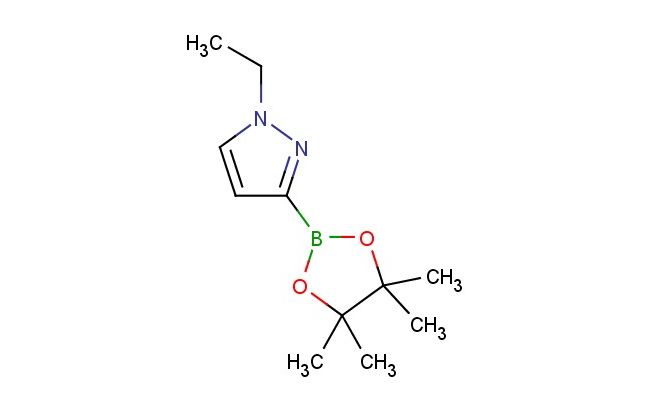
1-ethyl-3-(4,4,5,5-tetramethyl-[1,3,2]dioxaborolan-2-yl)-1H-pyrazole
$400.00
CAS No.: 1233525-88-6
Catalog No.: 195643
Purity: 95%
MF: C11H19BN2O2
MW: 222.097
Storage: 2-8 degree Celsius
SMILES: C(C)N1N=C(C=C1)B1OC(C(O1)(C)C)(C)C
Catalog No.: 195643
Purity: 95%
MF: C11H19BN2O2
MW: 222.097
Storage: 2-8 degree Celsius
SMILES: C(C)N1N=C(C=C1)B1OC(C(O1)(C)C)(C)C
1-ethyl-3-(4,4,5,5-tetramethyl-[1,3,2]dioxaborolan-2-yl)-1H-pyrazole; CAS No.: 1233525-88-6; 1-ethyl-3-(4,4,5,5-tetramethyl-[1,3,2]dioxaborolan-2-yl)-1H-pyrazole. PROPERTIES: 1-Ethyl-3-(4,4,5,5-tetramethyl-[1,3,2]dioxaborolan-2-yl)-1H-pyrazole has molecular formula C12H19BNO4, giving it a molecular weight of 258.2 g/mol. It appears as a pale yellow liquid with a boiling point between 95-100 C at 10 mmHg. The compound exhibits moderate solubility in polar aprotic solvents and is air-sensitive due to the boronate ester group. Recommended storage involves keeping it in nitrogen or argon atmosphere at -20 C. Safety precautions include using powder-free gloves and avoiding contact with water, as hydrolysis may release boric acid. The compound has a vapor pressure of approximately 0.1 mmHg at 25 C and a density of 1.18 g/mL. APPLICATIONS: This 1-ethyl-3-(4,4,5,5-tetramethyl-[1,3,2]dioxaborolan-2-yl)-1H-pyrazole is a valuable intermediate in modern pharmaceutical synthesis, particularly in Suzuki-Miyaura coupling reactions. The boronate ester group enables efficient cross-coupling with aryl halides to form biaryl pyrazoles, which are common scaffolds in kinase inhibitors. A comprehensive review in Organic Process Research & Development highlighted its role in developing anticancer agents targeting EGFR kinases in non-small cell lung cancer. In chemical research, it serves as a protected pyrazole building block. The tetramethyl dioxaborolane group provides temporary protection while allowing selective functionalization at other positions. Additionally, the compound is utilized in the preparation of PET imaging agents. Research in Bioconjugate Chemistry demonstrated its utility in creating boronate-containing radiotracers for monitoring glucose metabolism in neurological disorders. The ethyl substituent enhances solubility in organic solvents, facilitating purification steps in multistep syntheses.
Reviews
Write Your Own Review