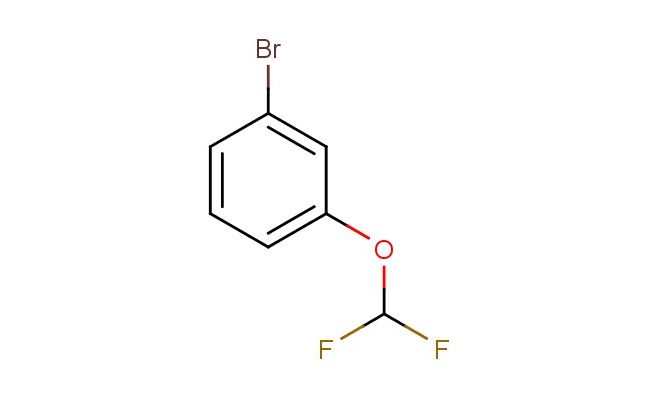
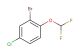
1-bromo-3-(difluoromethoxy)benzene
$250.00
CAS No.: 262587-05-3
Catalog No.: TQU0263
Purity: 95%
MF: C7H5BrF2O
MW: 223.016
Storage: 2-8 degree Celsius
SMILES: BrC1=CC(=CC=C1)OC(F)F
Catalog No.: TQU0263
Purity: 95%
MF: C7H5BrF2O
MW: 223.016
Storage: 2-8 degree Celsius
SMILES: BrC1=CC(=CC=C1)OC(F)F
For R&D use only. Not for human or animal use.
CAS NO.: 262587-05-3; 1-bromo-3-(difluoromethoxy)benzene. PROPERTIES: 1-bromo-3-(difluoromethoxy)benzene appears as colorless liquid with a mild ether-like odor. Its molecular formula is C7H5BrF2O, corresponding to a molecular weight of 213.02 g/mol. The compound has a density of approximately 1.55 g/cm? and a boiling point around 145 C at 760 mmHg. It is moderately soluble in ether and chlorinated solvents but has limited water solubility. Proper storage requires temperatures of 2-8 degree Celsius in glass containers with Teflon-lined caps to prevent degradation. When handling, use chemical-resistant gloves and eye protection to prevent skin and eye contact, which may cause mild irritation. The substance is stable under dry conditions but undergoes hydrolysis in basic environments, releasing the corresponding phenol and hydrogen bromide. It is classified as a mild irritant and should be managed in well-ventilated areas. APPLICATIONS: 1-bromo-3-(difluoromethoxy)benzene serves as a versatile building block in organic synthesis. The bromine substituent allows for cross-coupling reactions, enabling the introduction of diverse aryl and heteroaryl groups. The difluoromethoxy group provides unique electronic effects that modulate the reactivity of the aromatic ring in subsequent transformations. In pharmaceutical research, this compound is used to develop kinase inhibitors where the fluoro substituents enhance binding affinity to the ATP-binding pocket. The bromine group facilitates Suzuki-Miyaura couplings, allowing for the introduction of aryl groups that improve pharmacokinetic properties. In materials science, derivatives of this compound are employed in the synthesis of liquid crystal displays where the difluoromethoxy group contributes to desirable electro-optical properties. Researchers in agrochemical development utilize this compound (though not for agricultural applications) to synthesize herbicidal agents with novel modes of action, exploiting the compound's ability to modulate plant enzyme activities. Additionally, the compound functions as a starting material for synthesizing fluorescent probes with blue-shifted emission profiles, useful in cellular imaging applications where low autofluorescence is critical.
Reviews
Write Your Own Review