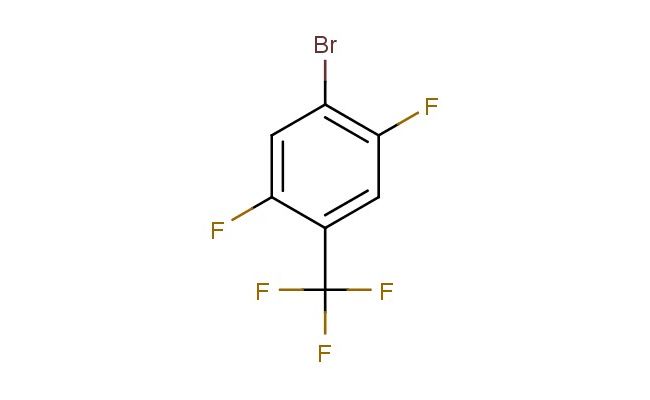
1-bromo-2,5-difluoro-4-(trifluoromethyl)benzene
$250.00
CAS No.: 261945-75-9
Catalog No.: WLZ1576
Purity: 95%
MF: C7H2BrF5
MW: 260.987
Storage: 2-8 degree Celsius
SMILES: BrC1=C(C=C(C(=C1)F)C(F)(F)F)F
Catalog No.: WLZ1576
Purity: 95%
MF: C7H2BrF5
MW: 260.987
Storage: 2-8 degree Celsius
SMILES: BrC1=C(C=C(C(=C1)F)C(F)(F)F)F
CAS NO.: 261945-75-9; 1-bromo-2,5-difluoro-4-(trifluoromethyl)benzene. PROPERTIES: This halogenated aromatic compound features a bromine atom, two fluorine atoms, and a trifluoromethyl group on a benzene ring, creating a molecule with potential applications in organic synthesis and pharmaceutical research. The 1-bromo-2,5-difluoro-4-(trifluoromethyl)benzene typically appears as a colorless to pale yellow liquid with moderate solubility in common organic solvents. Its molecular structure includes multiple halogen atoms and a trifluoromethyl group that influence the electronic properties of the aromatic system. For optimal stability and to prevent premature reactions, this compound should be stored at 2-8 degree Celsius in an amber glass bottle under an inert atmosphere. When handling, appropriate safety measures including nitrile gloves and safety goggles are recommended. This compound is sensitive to light and moisture, requiring careful environmental control during storage and use. In case of skin contact, wash thoroughly with soap and water; if eye contact occurs, rinse immediately and seek medical evaluation. APPLICATIONS: The 1-bromo-2,5-difluoro-4-(trifluoromethyl)benzene serves as a versatile building block in organic synthesis, particularly for creating halogenated bioactive molecules. The bromine substituent provides a handle for cross-coupling reactions, enabling the creation of substituted benzene derivatives with diverse biological activities. In medicinal chemistry, this compound functions as an intermediate for developing pharmaceuticals targeting enzyme inhibitors and receptor modulators. The trifluoromethyl group contributes to target binding affinity and selectivity. Additionally, the molecule finds utility in materials science as a monomer for creating polymers with specific electronic and optical properties. Researchers utilizing this compound can benefit from its functional group versatility, enabling the development of diverse molecular architectures for applications ranging from drug discovery to advanced materials.
Reviews
Write Your Own Review