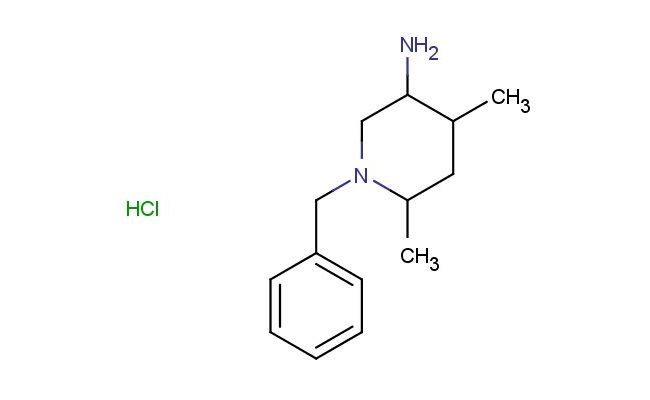
1-benzyl-4,6-dimethylpiperidin-3-amine hydrochloride
$275.00
CAS No.: 2682114-52-7
Catalog No.: 192839
Purity: 95%
MF: C14H23ClN2
MW: 254.805
Storage: 2-8 degree Celsius
SMILES: Cl.C(C1=CC=CC=C1)N1CC(C(CC1C)C)N
Catalog No.: 192839
Purity: 95%
MF: C14H23ClN2
MW: 254.805
Storage: 2-8 degree Celsius
SMILES: Cl.C(C1=CC=CC=C1)N1CC(C(CC1C)C)N
For R&D use only. Not for human or animal use.
1-benzyl-4,6-dimethylpiperidin-3-amine hydrochloride; CAS No.: 2682114-52-7; 1-benzyl-4,6-dimethylpiperidin-3-amine hydrochloride. PROPERTIES: This benzyl- and dimethyl-substituted piperidine amine salt has molecular formula C15H22N2 {HCl. It appears as a white crystalline powder. The 1-benzyl-4,6-dimethylpiperidin-3-amine hydrochloride exhibits high water solubility (exceeding 100 mg/mL) and moderate solubility in common polar solvents. Its melting point ranges between 180-185 C (with decomposition), and it has a molecular weight of approximately 266.82 g/mol (free base). When handling, care should be taken to avoid skin contact and use of proper respiratory protection. Storage should be in a tightly sealed container at room temperature, protected from light and moisture. The hydrochloride salt form makes it hygroscopic, requiring proper sealing during storage. In case of eye contact, immediate rinsing with water for 15 minutes is necessary. APPLICATIONS: The 1-benzyl-4,6-dimethylpiperidin-3-amine hydrochloride serves as a valuable intermediate in the synthesis of dual orexin receptor antagonists for insomnia treatment where the piperidine ring provides essential hydrogen bonding interactions with receptor subtypes (as detailed in medicinal chemistry literature). The benzyl and methyl groups enhance metabolic stability by preventing oxidative degradation. Additionally, the compound functions as a building block in the preparation of bioconjugates for drug delivery systems where the amine groups react with targeting moieties, as described in pharmaceutical sciences journals. The amine groups can be further functionalized through acylation or sulfonamidation reactions to produce various derivatives for chemical research applications.
Reviews
Write Your Own Review